Contents
- Inflammation
- Infection
- Vascular changes
- Tissue responses to injury
- Pathology of tumours
- Classification of tumours
- Multidisciplinary team meetings (MDTs)
- Autoimmune disorders
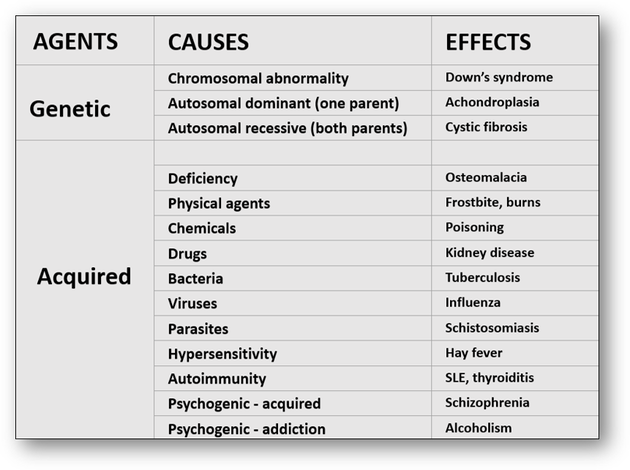
Cellular pathology is defined as the diagnosis of disease that deals specifically with the histological examination of cells and tissues. Disease processes arise from disturbances of function and structure of cells and tissues and are broadly classified into acquired (which develop as a result of environmental factors) and genetic, which arise from abnormalities of base sequences in DNA (Figure 1).
If cell death is rapid, little or no histological changes are apparent. Conversely, if a patient survives for several hours, changes subsequent to cell death occur and these may be visible both macroscopically and microscopically.
1. Inflammation
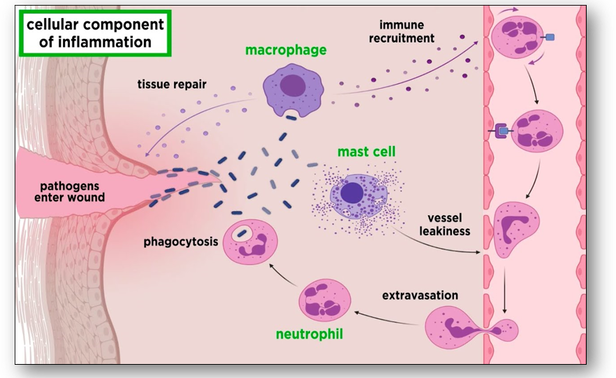
When living tissues are injured, a series of changes occur in and around the area of injury. This response to injury is known as inflammation, and although the injury is abnormal, inflammation is a normal physiological reaction (Figure 2). The inflammatory nature of a lesion is usually indicated by the suffix ‘itis’ (such as appendicitis) and there are several basic signs of the inflammation process that may be observed:
- Redness - due to dilatation of small blood vessels within the injured area
- Heat - due to increased local blood flow when the inflamed area is near skin
- Swelling - local oedema caused by accumulation of fluid exudate
- Pain - caused by a rise in tissue tension over oedematous areas
- Loss of function - as a result of inhibition of muscle movement as a result of pain and swelling
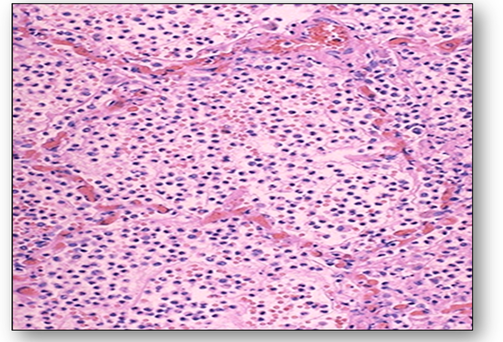
When bacteria and other pathogens enter a wound, platelets form blood-clotting proteins at the site of injury. The mast cells secrete factors that mediate vasodilation and an increase in delivery of blood plasma and cells occur. One of the most prominent histological feature of inflammation is the migration of leucocytes through capillary walls and their subsequent movement in the tissues. These cells play a role in the digestion and phagocytosis of fibrin, degenerate tissue and bacteria. This helps to eliminate invasive bacteria and also limits injurious effects of chemicals and toxins by carrying them away via the lymphatics. Inflammatory exudate is produced which flows through inflamed tissues, helping to dilute the toxins. Antibodies are also produced and these react with and destroy the source of infection. The production of a fibrin network forms a mechanical barrier. The inflammatory response continues until the foreign material is eliminated and the wound is repaired. Acute inflammation is an immediate, adaptive response and the section of lung below shows acute bronchopneumonia with numerous neutrophils filling the alveoli of the lung (Figure 3). This patient would have had a productive cough as a result of the large amounts of purulent sputum produced. Acute inflammation can lead to several outcomes :
When bacteria and other pathogens enter a wound, platelets form blood-clotting proteins at the site of injury. The mast cells secrete factors that mediate vasodilation and an increase in delivery of blood plasma and cells occur. One of the most prominent histological feature of inflammation is the migration of leucocytes through capillary walls and their subsequent movement in the tissues. These cells play a role in the digestion and phagocytosis of fibrin, degenerate tissue and bacteria. This helps to eliminate invasive bacteria and also limits injurious effects of chemicals and toxins by carrying them away via the lymphatics. Inflammatory exudate is produced which flows through inflamed tissues, helping to dilute the toxins. Antibodies are also produced and these react with and destroy the source of infection. The production of a fibrin network forms a mechanical barrier. The inflammatory response continues until the foreign material is eliminated and the wound is repaired. Acute inflammation is an immediate, adaptive response and the section of lung below shows acute bronchopneumonia with numerous neutrophils filling the alveoli of the lung (Figure 3). This patient would have had a productive cough as a result of the large amounts of purulent sputum produced. Acute inflammation can lead to several outcomes :
- Resolution, where tissue reverts to normal as the inflammatory changes subside.
- Progression to suppuration, which involves the digestion of dead tissue by leucocytes. This generates spaces full of exudate that contains leucocytes, bacteria, necrotic tissue and fibrin. This is termed an abscess cavity and can contain fluid (pus) that may become creamy due to the high nucleic acid content from the dead leucocytes. The return of the tissue to normal is not possible since the tissue is destroyed and the abscess becomes enclosed by granulation tissue that matures to form a scar.
- Progression to chronic phase with fibrosis. Chronic inflammation is the result of prolonged tissue injury where the time limit will depend on the nature of disease. This can be caused by agents such as bacteria, fungi, and particulate material such as asbestos fibres.
2. Infection
2. Infection
Infection is recognized as the invasion of the body by disease-causing agents collectively known as microbes or microorganisms. In the routine histology laboratory, these agents are typically identified as bacteria, fungi and viruses although protozoa and helminths may be seen less frequently. Histological evaluation of tissue sections by the application of controlled staining methods is a simple way of identifying agents responsible for infection. For example, the Gram stain traditionally classifies bacteria as Gram positive or Gram negative depending on the staining reaction. More specifically, identification of the organisms responsible for tuberculosis can be achieved using an acid fast staining method such as the Ziehl Neelsen (ZN) stain. Similarly, positive recognition of fungi is possible using routine methods such as periodic acid Schiff (PAS) and methenamine silver. In viral infections such as rabies, inclusion bodies may appear within the cells during multiplication of the virus. Although these are often visualized with haematoxylin and eosin and Giemsa-type stains, immunohistochemistry and molecular hybridization are the preferred methods for virus identification. Medically important helminths and protozoa depend upon invasion of a suitable host to complete all or part of their life cycle and as such are termed parasites. While protozoa are associated with diseases such as amoebic dysentery and malaria, helminths such as the parasitic worms are responsible for global diseases that far exceed that of malaria and tuberculosis by currently infecting over a billion people worldwide. In the laboratory, these medical parasites can be identified microscopically using a variety of methods such as haematoxylin and eosin and trichrome stains. The classification and pathological significance of infective agents encountered are many and are subdivided into the following groups: Bacteria, fungi, algae, protozoa, helminths and viruses.
Bacteria
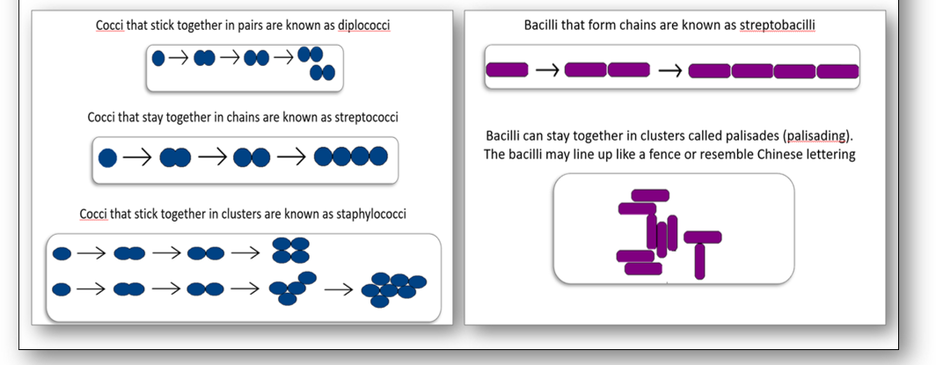
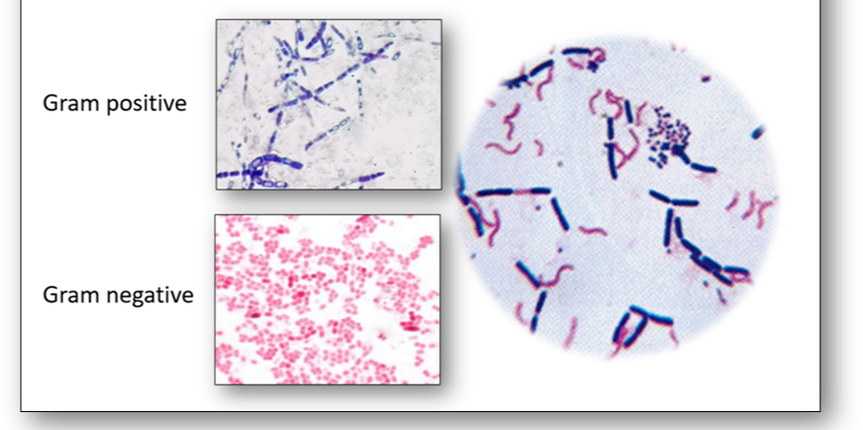
The morphology or shape of a bacteria cell is the most characteristic property which can also decide its pathogenicity (Figure 4). Most bacteria contain genetic information in a single loop of DNA and are classified according to their basic shapes which are spherical (cocci) or rods (bacilli). They can exist as single cells, in pairs, chains or clusters and the bacilli may also be spiral, corkscrew or comma shaped.
One of the most common bacterial stains encountered in the histology laboratory is the Gram stain which is used to stain bacteria to determine if they are Gram positive (purple) or Gram negative (red). The primary stain is crystal violet which differentiates bacteria based upon the properties of their cell wall, specifically the thickness of the peptidoglycan layer. Bacteria that have a thin layer of peptidoglycan do not retain the primary stain which washes out when ethanol is added. The safranin counterstain consequently stains the Gram negative cells pink to red (Figure 5).
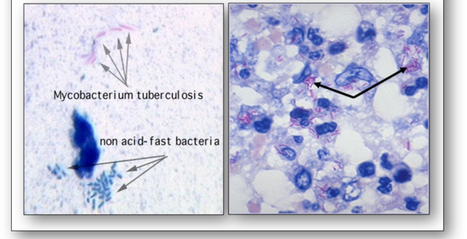
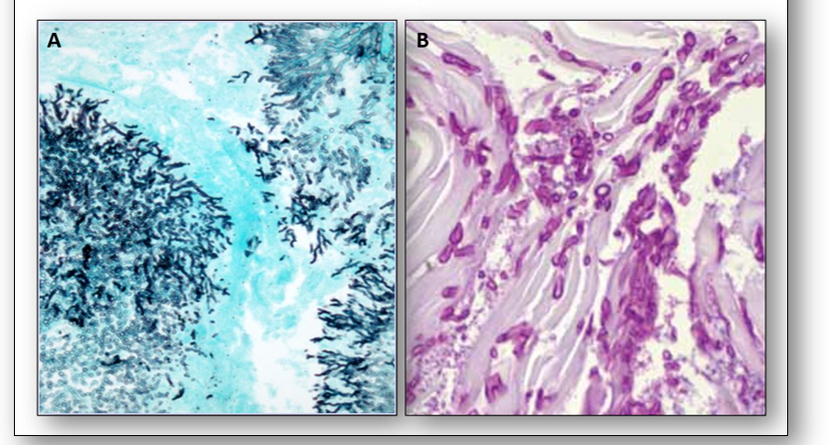
Another widely used technique for certain bacteria is acid-fast staining (Figure 6). The methods of Ziehl Neelsen (ZN) and Kinyoun (cold ZN) are used to demonstrate cysts of Cryptosporidium and certain other bacteria which do not stain well with Gram (such as Mycobacteria tuberculosis) because their walls are impermeable to the dyes. The cell walls of mycobacteria have a high lipid content (primarily mycolic acid) which the carbol fuchsin stain will bind to. Although this stain can be removed with an acid-alcohol decolourizer, stained cells that retain the dye are termed acid-fast and will appear red-purple (Figure 7). Conversely, cells that release the primary stain on decolourizing are termed non acid-fast and will appear green or blue depending upon the counterstain used.
Other bacteria
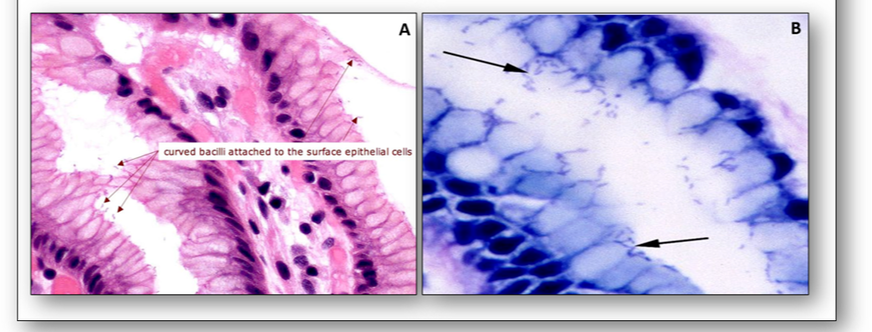
Helicobacter pylori are flagellated, ‘S’-shaped, Gram-negative bacilli and are the cause of most gastric and duodenal ulcers. They are visible with the haematoxylin and eosin stain and Giemsa type methods (Figure 8).
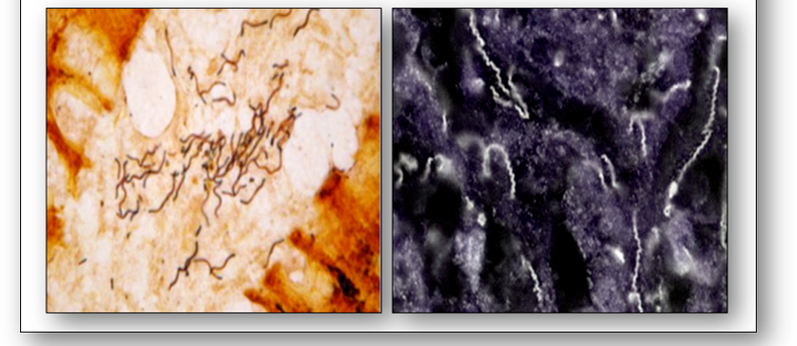
Spirochaetes are spiral in shape and generally Gram negative. They also stain with a variety of silver methods such as that of Steiner and by dark field microscopy (Figure 9). Spirochaetes cause diseases such as syphilis (Treponema pallidum) and Lyme disease (Borrelia burgdorferi).
Fungi
Fungi include molds, yeasts and higher fungi. Their cell walls are composed of the polysaccharide cellulose but unlike bacteria, they do not contain peptidoglycan. In tissues, fungi usually occur as hyphae, budding yeast spherules or a combination and provide evidence of infection. Evaluation of granulomatous inflammation must include special stains to exclude presence of fungi (and acid-fast bacteria). Methenamine silver and Gridley’s modified PAS method are the most common staining methods (Figure 10).
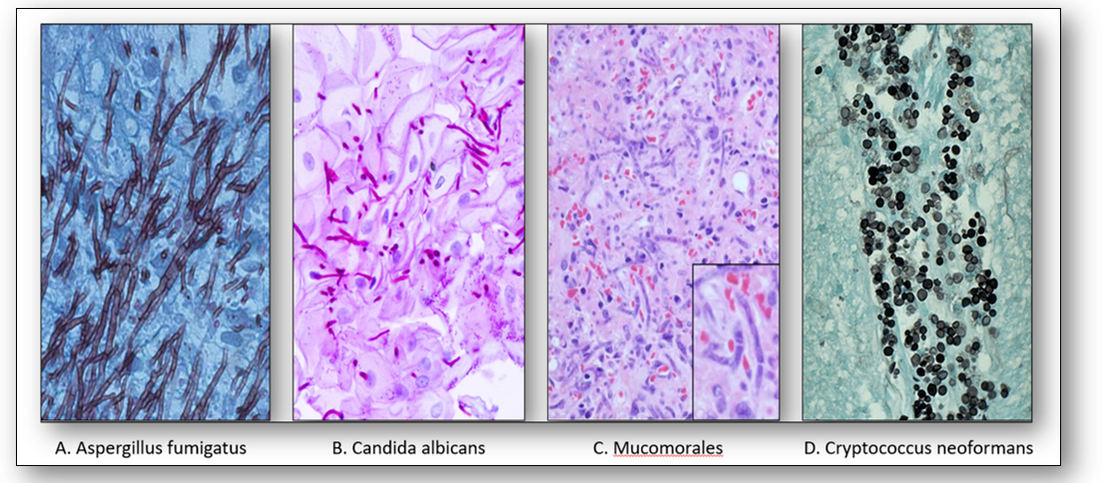
Other important pathogenic fungi are demonstrated below (Figure 11). These include Aspergillus fumigatus, a mold that causes aspergillosis (A); Candida albicans, an opportunistic pathogenic yeast that causes thrush, an infection affecting the vagina, penis and skin (B); Mucomorales, the causative agent of mucormycosis, a serious but rare fungal infection caused by environmental molds (C); and Cryptococcus neoformans which causes infections of the lung and encephalitis (D). This fungus is found in bird droppings and soil and spreads by the inhalation of spores.
Other important pathogenic fungi are demonstrated below (Figure 11). These include Aspergillus fumigatus, a mold that causes aspergillosis (A); Candida albicans, an opportunistic pathogenic yeast that causes thrush, an infection affecting the vagina, penis and skin (B); Mucomorales, the causative agent of mucormycosis, a serious but rare fungal infection caused by environmental molds (C); and Cryptococcus neoformans which causes infections of the lung and encephalitis (D). This fungus is found in bird droppings and soil and spreads by the inhalation of spores.
Algae
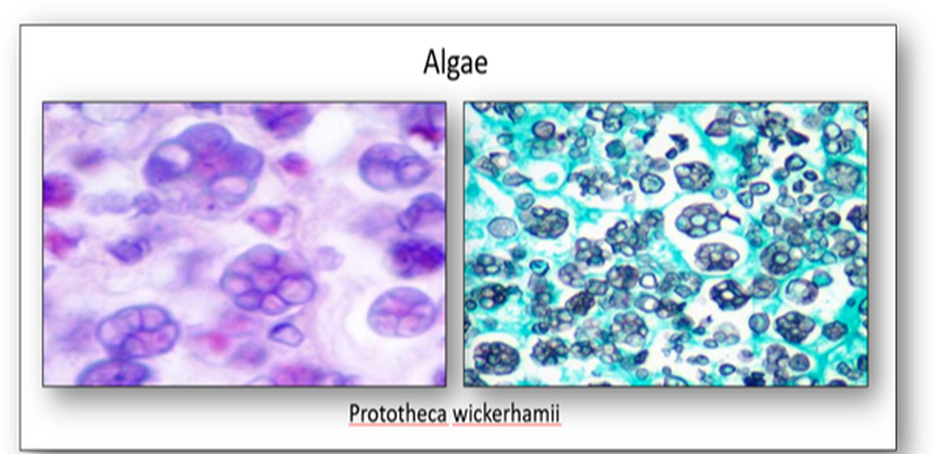
The only algae to infect humans is Prototheca wickerhamii, the causative agent of protothecosis (Figure 12). An infection that occurs via inoculation of skin following exposure to contaminated water or tree slime, it also affects cats, dogs and cattle.
Protozoa
These are a diverse group of unicellular organisms, many of which are motile. Restricted to moist or aquatic habitats, there are an estimated 30,000 protozoan species, many of which are parasitic. Protozoa are generally classified as intestinal, urogenital and blood and tissue.
Intestinal protozoa (see Figure 13)
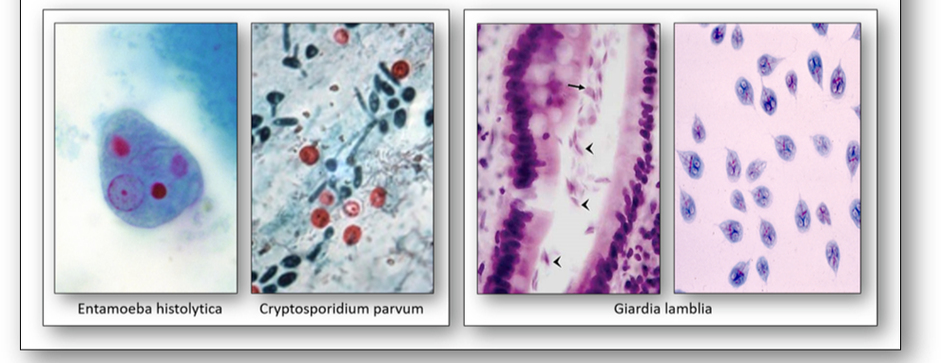
- Entamoeba histolytica causes amoebic dysentery in travellers to endemic areas. The infection can cause liver abscesses and the protozoa can be demonstrated using a trichrome stain.
- Cryptosporidium parvum causes the watery diarrhoea disease known as cryptosporidiosis. Immunodeficient patients may be unable to clear the parasite and it can prove fatal. This organism can be demonstrated with the Kinyoun stain (cold ZN).
- Giardia lamblia is a protozoa that infects the bowel and causes giardiasis which can lead to chronic diarrhoea. Spread by water contaminated with infected faeces, it is commonly found in countries with poor sanitation (beaver fever). In sections stained with H&E, it has the appearance of tumbling leaves.
Urogenital protozoa
- Trichomonas vaginalis is a primitive eukaryotic flagellate organism that causes trichomoniasis. The disease is sexually transmitted and a common cause of genitourinary tract infections such as urethritis and vaginitis. They can be readily identified in Papanicolaou stained cervical smears (Figure 14).
Blood and tissue protozoa (see Figure 15)
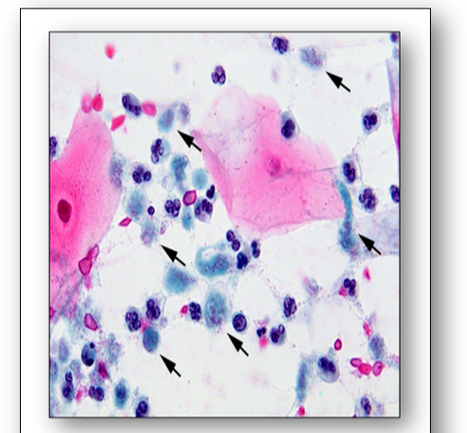
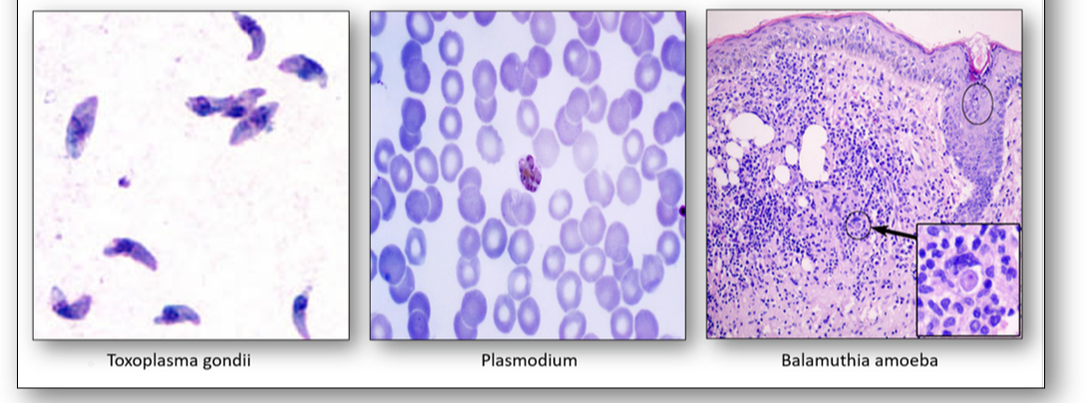
- Toxoplasma gondii can cause congenital disorders from acute infection of the mother during pregnancy. Babies may have significant pathology in later life. Cats are a natural host.
- Plasmodium are a taxonomic group of single-celled parasites with characteristic secretory organelles at one end of the cell. They are carried by mosquitoes and cause malaria. There are 4 main species of Plasmodium: vivax, malariae, ovale and falciparum.
- Balamuthia amoeba are found in the environment and consist of a cystic and non-cystic stage. They can form a skin lesion or migrate to the brain where they may cause granulomatous amoebic encephalitis (GAE) which can result in facial paralysis, seizures and death.
Helminths
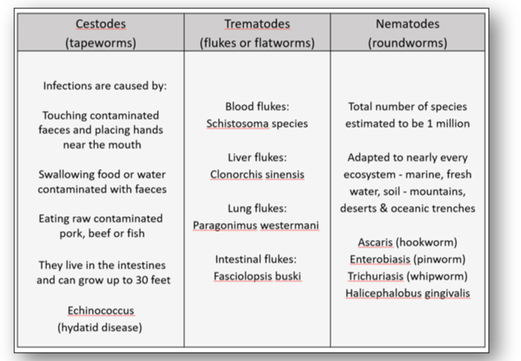
Also known as parasitic worms, these are large macroparasites which in the mature form can often be seen with the naked eye. Many are intestinal worms that are soil-transmitted and infect the gastrointestinal tract. Other parasitic worms such as schistosomes reside in blood vessels. Helminths are generally grouped as cestodes (tapeworms), trematodes (flukes or flatworms) and nematodes (roundworms) - see Figure 16a.
Cestodes
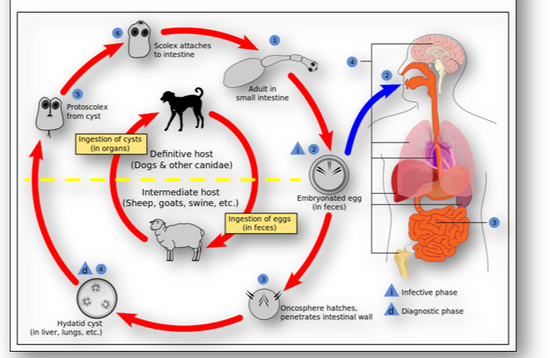
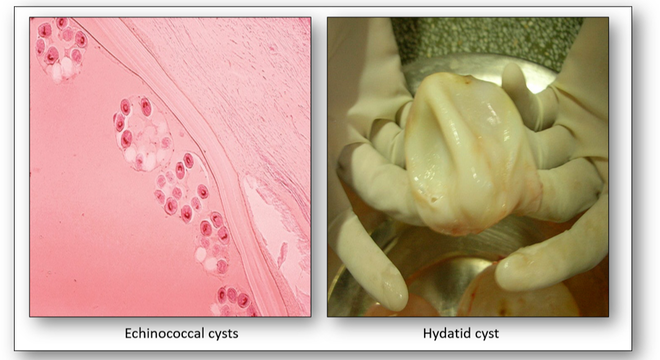
Echinococcus granulosus (hydatid or dog tapeworm) dwells in the small intestine of dogs as an adult. The worm has important intermediate hosts such as livestock and humans, where it causes cystic echinococcosis (hydatid disease). The disease spreads when food or water containing the eggs of the worm are eaten or by close contact with an infected animal (see Figure 16b). The eggs are then released in the faeces of infected meat-eating animals such as dogs and foxes. For these animals to become infected they must eat the organs of an animal that contains the cysts (Figure 16c).
Trematodes
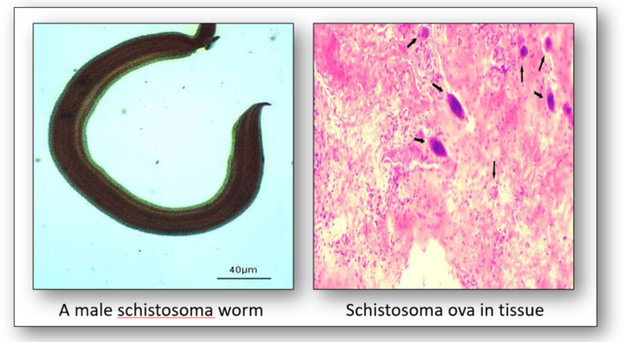
The genus Schistosoma comprises more than 20 recognized species which are divided into four groups: indicum, japonicum, mansoni and haematobium (Figure 17). The worm causes schistosomiasis (snail fever or bilharzia) by infecting the urinary tract and intestines and causing liver and kidney damage. The disease is spread by contact with water containing the parasites which are released from infected freshwater snails. The disease affects one fifth of sub-Saharan Africa.
The genus Schistosoma comprises more than 20 recognized species which are divided into four groups: indicum, japonicum, mansoni and haematobium (Figure 17). The worm causes schistosomiasis (snail fever or bilharzia) by infecting the urinary tract and intestines and causing liver and kidney damage. The disease is spread by contact with water containing the parasites which are released from infected freshwater snails. The disease affects one fifth of sub-Saharan Africa.
Nematodes
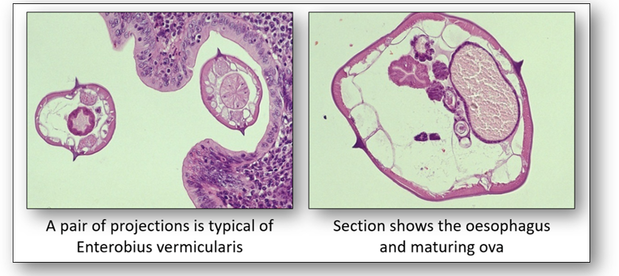
There are more than 28,000 species of Nematodes identified. The roundworm Enterobius vermicularis is also known as the pin or threadworm and is the most common intestinal parasite in the western world (Figure 18). It causes enterobiasis which is frequent among people in close contact. In children, it is spread by finger sucking and nail biting.
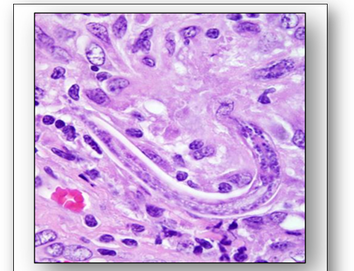
Halicephalobus gingivalis, a nematode that lives actively in soil, plants, manure and compost is a parasite of horses (Figure 19). It may be transmitted between humans via organ transplant and cause inflammation of the central nervous system which often leads to meningoencephalitis, an often fatal disease.
Halicephalobus gingivalis, a nematode that lives actively in soil, plants, manure and compost is a parasite of horses (Figure 19). It may be transmitted between humans via organ transplant and cause inflammation of the central nervous system which often leads to meningoencephalitis, an often fatal disease.
Viruses
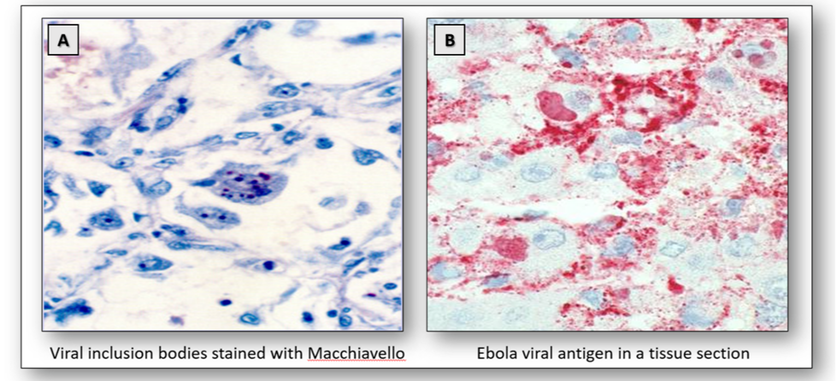
Viral inclusion bodies are abnormal structures that appear within the cell nuclei, cytoplasm or both during virus multiplication. The presence of inclusion bodies may often be seen in sections stained with H&E but special stains such as Giemsa or Macchiavello may be carried out (Figure 20A). However, antibody immunohistochemistry or hybridization with molecular probes are the preferred methods. There are several viruses that are of histological interest, and these are described below.
Ebola virus is carried by fruit bats and causes haemorrhagic fever. The disease is spread by direct contact with body fluids and characteristic cytoplasmic inclusions can be seen on H&E. Ebola viral antigens can also be demonstrated in tissue sections using immunohistochemistry (Figure 20B).
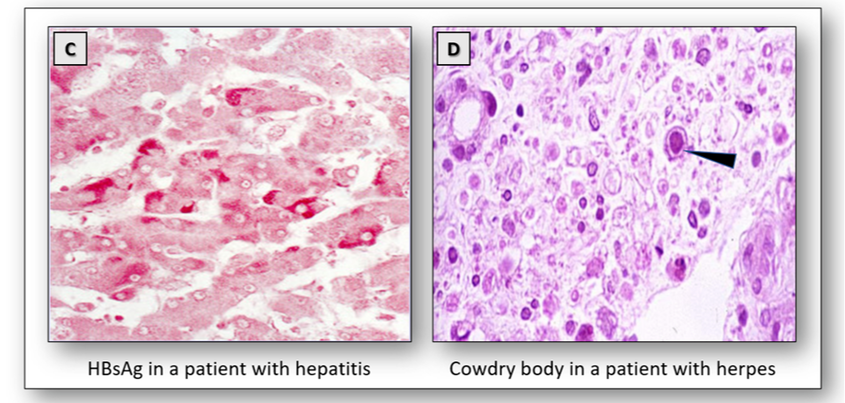
The surface antigen of the hepatitis B virus (HBsAg) indicates current hepatitis B infection. Known as Australia antigen, it was first demonstrated in an Australian aborigine. The antigen can be stained with the Shikata orcein method (Figure 20C).
Herpes virus Type 1 produces recurrent blisters around the lips and generalized infections in older children and adults while Type 2 affects the genital tract. Inclusion bodies do not appear until after the virus has multiplied. In the H&E, Cowdry bodies appear as intranuclear eosinophilic inclusions in cells infected with the herpes virus (Figure 20D).
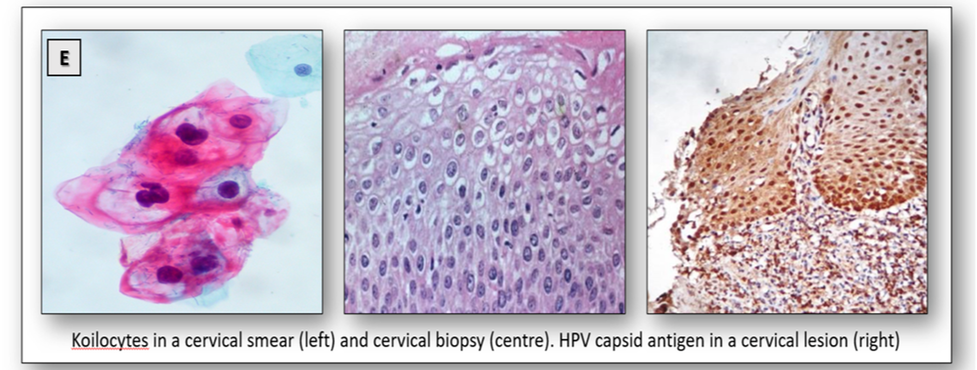
The human papillomavirus (HPV) is a circular, double-stranded DNA virus that induces warts on skin and mucosal surfaces. There are currently more than 200 related types of HPV with many of them known to cause genital disease. Infections are highly contagious and are the most common sexually transmitted disease causing genital warts and cervical cancer. Low risk HPV strains such as types 6 and 11 are known to cause about 90% of genital warts which rarely develop into cancer. However, there are a number of high risk types that are known to cause cancer of the cervix and premalignant lesions such as cervical intraepithelial neoplasia (CIN). In particular, the high risk HPV types 16 and 18 are associated with more than 75% of cervical cancer cases. The presence of koilocytes in cervical smears and biopsies can serve as a primary indicator of underlying infection, while immunostaining can identify the presence of viral antigen (Figure 20E). Using type specific viral probes, DNA technology is able to detect type-specific HPV genomes in infected tissues.
The human papillomavirus (HPV) is a circular, double-stranded DNA virus that induces warts on skin and mucosal surfaces. There are currently more than 200 related types of HPV with many of them known to cause genital disease. Infections are highly contagious and are the most common sexually transmitted disease causing genital warts and cervical cancer. Low risk HPV strains such as types 6 and 11 are known to cause about 90% of genital warts which rarely develop into cancer. However, there are a number of high risk types that are known to cause cancer of the cervix and premalignant lesions such as cervical intraepithelial neoplasia (CIN). In particular, the high risk HPV types 16 and 18 are associated with more than 75% of cervical cancer cases. The presence of koilocytes in cervical smears and biopsies can serve as a primary indicator of underlying infection, while immunostaining can identify the presence of viral antigen (Figure 20E). Using type specific viral probes, DNA technology is able to detect type-specific HPV genomes in infected tissues.
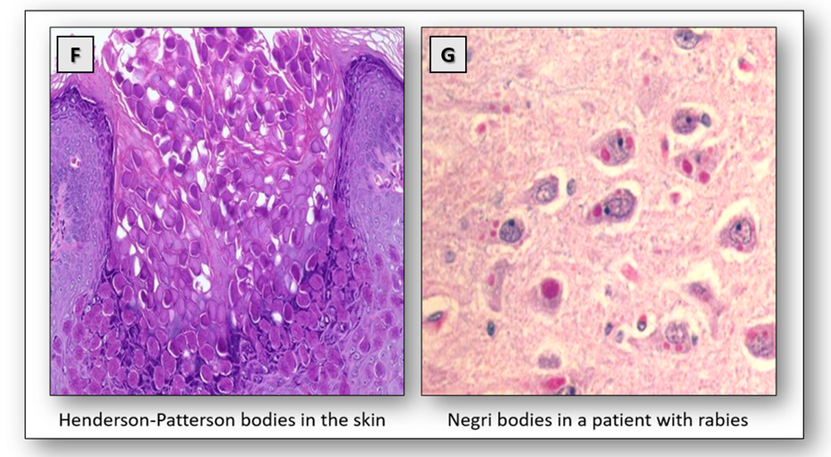
The poxvirus is known to cause molluscum contagiosum, a common non-cancerous growth. In this lesion, eosinophilic, cytoplasmic inclusions called Henderson-Patterson bodies can be seen in the cells of the uppermost layers of the skin. They consist of masses of maturing virus particles and can be seen in the H&E (Figure 20F).
The rabies virus is spread by bites from infected animals such as dogs, bats and monkeys. The presence of Negri bodies within the cytoplasm of nerve cells is diagnostic for rabies (Figure 20G).
3. Vascular changes
When tissue is first damaged, the blood vessels in the injured area constrict (vasoconstriction). Following this, the blood flow is increased when the vessels dilate (vasodilation) and the vessels also become more permeable, allowing the exudate (a protein-rich fluid which includes factors to help the blood to clot) to exit the tissues. This helps to prevent the spread of infectious agents and allows the antibody proteins to destroy the invading bacteria. The process that prevents bleeding is known as haemostasis and is the first stage of wound healing. This stage changes blood from a liquid phase to a gel and this is known as coagulation, The final product of the blood coagulation step is the formation of a blood clot or thrombus.
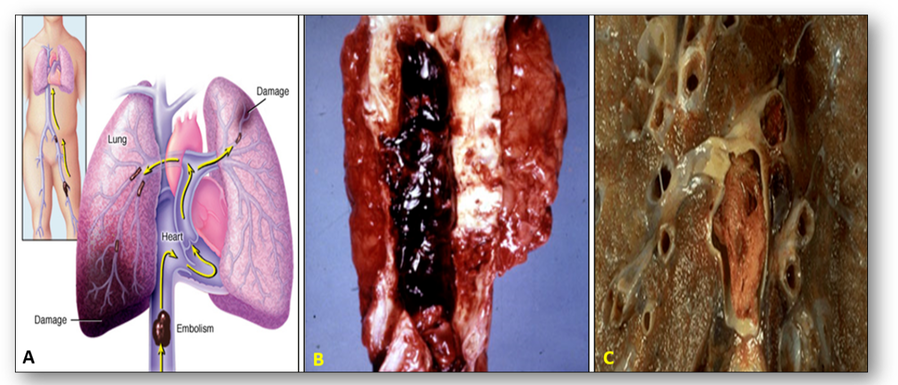
The condition in which blood flow is restricted or reduced in a part of the body is known as ischaemia, and the detached thrombus that causes the partial or complete obstruction is known as an embolism. In the image below, a pulmonary embolus can be seen traveling from a large vein in the leg to the main pulmonary arteries (Figure 21). Total occlusion of the artery by the embolus can occur and individuals who are immobilized are at greatest risk. Patients may experience a sudden onset of shortness of breath and death can occur within minutes.
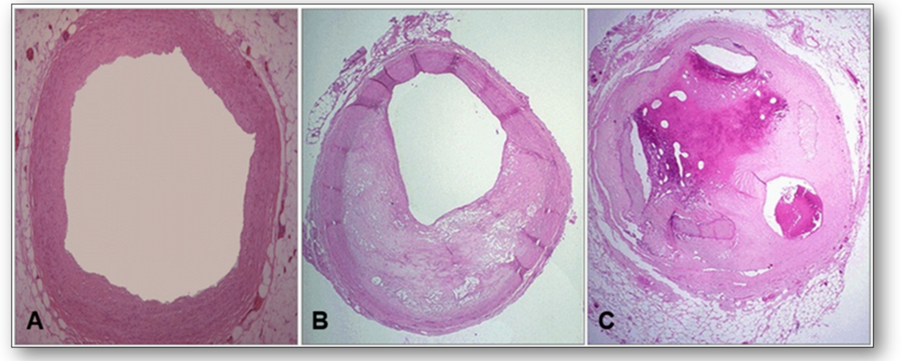
Disease of the arteries in which fatty material and plaque are deposited in the wall is known as atherosclerosis. A narrowing of the arterial lumen can be seen with impairment of blood flow (Figure 22).
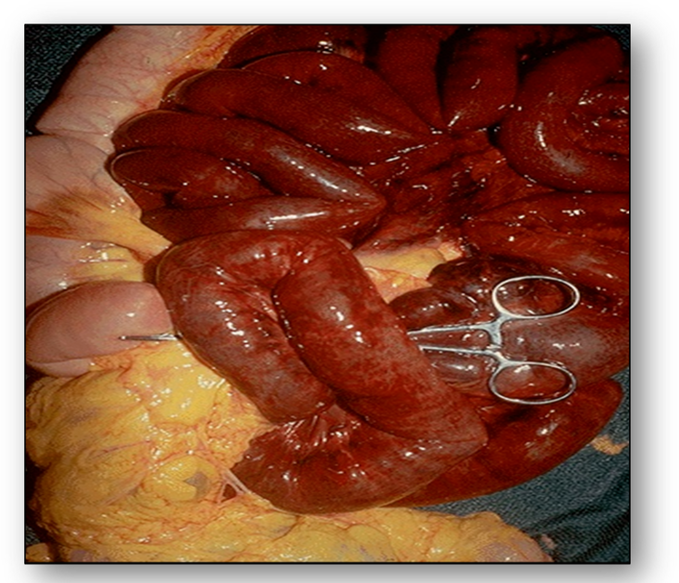
Interference with the arterial blood flow can exhibit localised areas of necrosis as a result of insufficient blood supply. This is termed infarction and can be seen as dark red infarcted bowel that contrasts with the pale pink normal bowel to the left of the image (Figure 23). This bowel was caught in a hernia and blood supply was constricted by the small opening to the hernia sac.
4. Tissue responses to injury
|
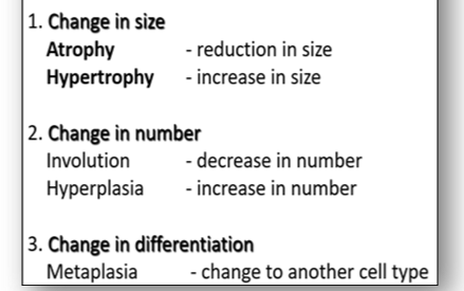
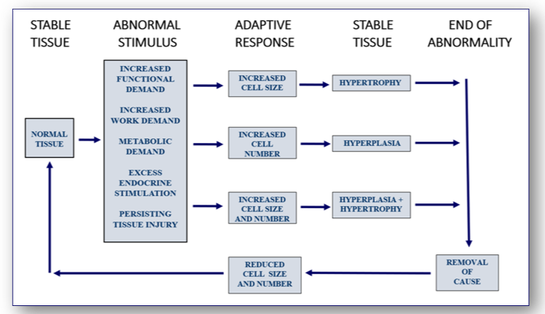
The development of cell and tissue growth is complex and is influenced by the combined effect of growth factors, hormones and the availability of nutrients. In disease processes, cells and tissues may go through abnormal changes in size, number and differentiation (Figure 24). For example, high blood pressure will increase the resistance of the heart to blood flow. This increase in functional demand will stimulate both hypertrophy and hyperplasia and result in enlargement of the myocardium (see below).
|
Responses resulting in increased tissue mass (see Figure 25)
Certain stimuli can affect cells and tissues by increasing demand on them. In the heart for example, further demand will increase the size of cardiac muscle fibres in response to the stimulus (Figure 26). Although the total number of muscle fibres does not increase, the response to the increased workload leads to marked thickening of the heart.
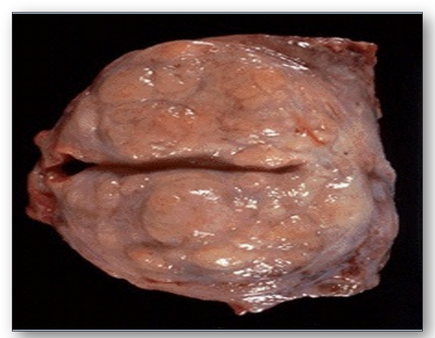
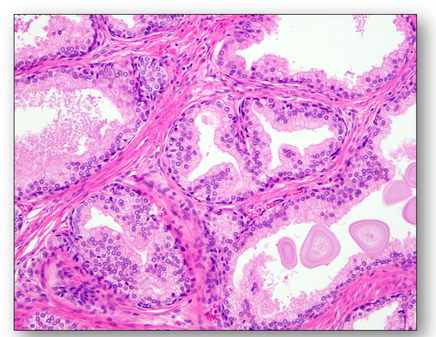
Hypertrophy of a prostate gland where the number of prostatic glands have increased in a nodular pattern (Figure 27). One of the prostatic nodules is shown in Figure 28, where the cells making up the glands are normal in appearance but there are just too many of them.
Hypertrophy of a prostate gland where the number of prostatic glands have increased in a nodular pattern (Figure 27). One of the prostatic nodules is shown in Figure 28, where the cells making up the glands are normal in appearance but there are just too many of them.
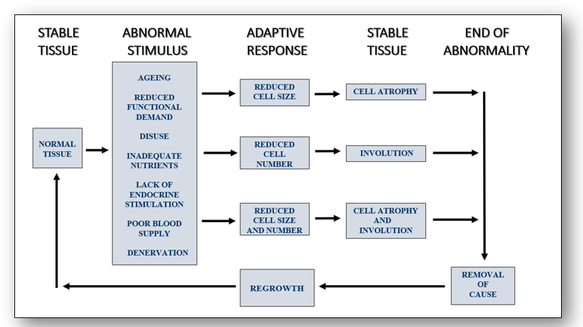
Responses resulting in decreased tissue mass (see Figure 29)
Responses resulting in decreased tissue mass (see Figure 29)
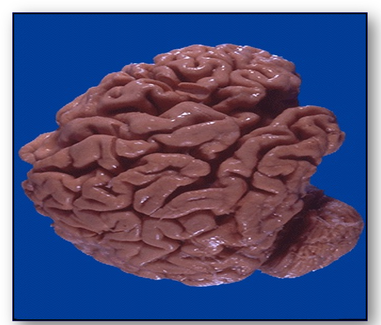
In Alzheimer's disease (Figure 30), the convolutions (gyri) of the brain below are narrowed while the furrows (sulci) are widened towards the frontal pole (cerebrum).
In the image below, atrophic muscle contains the same number of muscle cells as before the atrophy occurred. However, in response to injury, the downsizing of some fibres is reduced in order to conserve the cell (Figure 31).
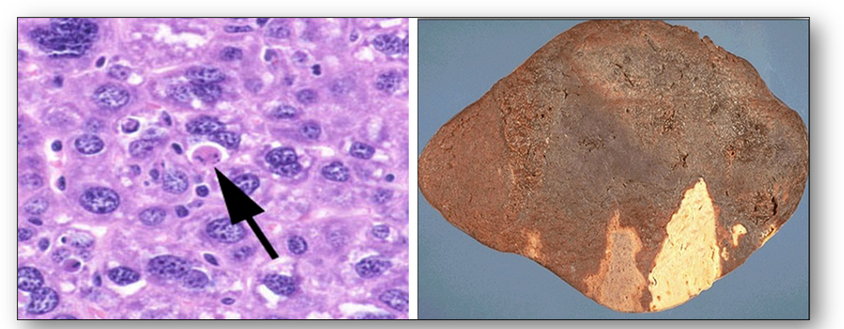
The double image above shows alcoholic liver changes (Figure 32). On the left, deranged transport of lipoprotein from injury (most often from alcoholism) leads to accumulation of lipid in the cytoplasm of hepatocytes. The image on the right shows cirrhosis of the liver with fibrosis and regeneration of the hepatocytes into nodules. Microscopically, the nodules show hepatocytes with pale areas of collagen in between them.
Cell death
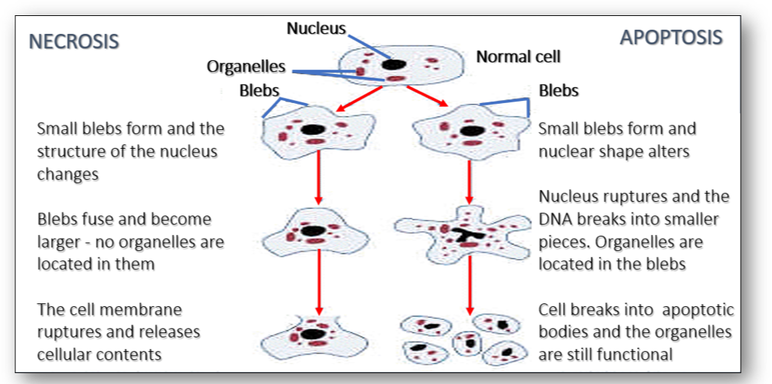
Cell death is the event of a biological cell ceasing to carry out its functions as a consequence of the natural process of old cells being replaced by new ones. Morphologically, cell death is generally classified into two distinct forms, namely apoptosis and necrosis (Figure 33). Apoptosis is a physiological process where cells that are no longer required commit suicide as a result of programmed events within the cells. Necrosis, however, is a non-physiological process that occurs as a result of infection or injury. The morphological differences of both types can be seen below. As well as apoptosis and necrosis, there are two other documented forms of cell death. Autophagy is a programmed cell death that differs from apoptosis, and the event known as entosis occurs when one cell inserts itself into an adjacent cell.
Cell death is the event of a biological cell ceasing to carry out its functions as a consequence of the natural process of old cells being replaced by new ones. Morphologically, cell death is generally classified into two distinct forms, namely apoptosis and necrosis (Figure 33). Apoptosis is a physiological process where cells that are no longer required commit suicide as a result of programmed events within the cells. Necrosis, however, is a non-physiological process that occurs as a result of infection or injury. The morphological differences of both types can be seen below. As well as apoptosis and necrosis, there are two other documented forms of cell death. Autophagy is a programmed cell death that differs from apoptosis, and the event known as entosis occurs when one cell inserts itself into an adjacent cell.
Apoptosis is a more orderly process in which there is individual cell death rather than death of large numbers of cells. In the image below, the apoptotic cell is pink and without a nucleus (Figure 34). Necrosis however, is pathological and invariably due to external factors. Since coagulative necrosis is usually vascular with loss of blood supply, the infarcts occur in a vascular distribution and are often wedge-shaped with a base on the capsule, as seen in the right hand image of a spleen below.
5. Pathology of tumours
Healthy cells are an essential requirement for normal bodily functions and depending on cell type they may be uniform or vary in both size and shape. When they are required to produce more healthy cells, they divide in a well-disciplined and organised manner. In disease processes, the balance becomes interrupted by biological changes which often allow malfunctions to develop (Figure 35). When cell division becomes uncontrolled, abnormal development occurs resulting in the formation of new growths (neoplasias) or tumours. Production of tumours exceeds the rate of cell death and they continue to grow indefinitely regardless of normal control mechanisms. The rate of tumour growth depends on several factors which include a supply of nutrients such as blood, and the number of dividing cells as opposed to the number of cells that have already divided or differentiated. Other mechanisms such as enzyme proteases have also been implicated in the growth and progression of tumour cells since these enzymes have the ability to degrade certain extracellular components.
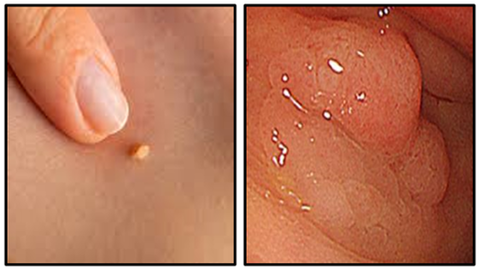
Tumours are broadly classified into two main categories: benign and malignant. Benign tumours usually comprise a single mass containing cells that closely resemble normal ones (Figure 36). They are generally symptomless, have well-defined margins and invariably remain at the site of origin. The reason for this is that the surface interaction molecules that hold tissues together are able to keep benign, normal appearing cells localized to appropriate tissues. Consequently, these masses can usually be excised completely. Benign tumours are seldom fatal unless they arise adjacent to and press on vital structures or secrete excess amounts of biologically active substances such as hormones.
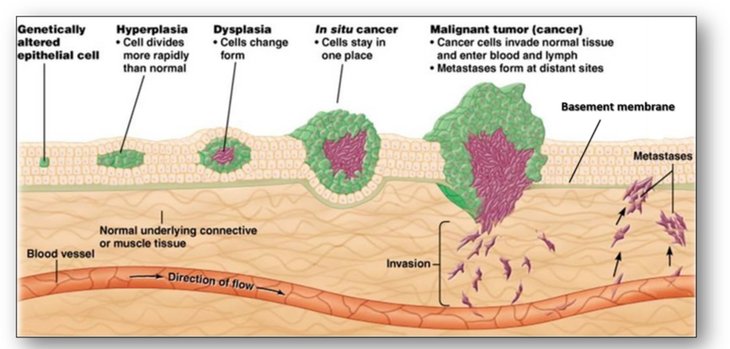
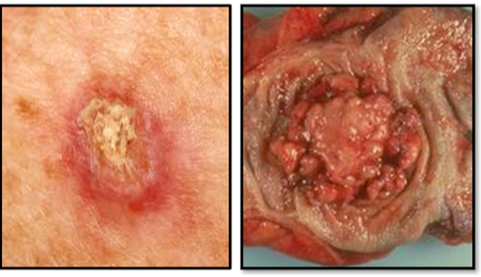
Conversely, the margins of a malignant tumour are poorly defined. The neoplastic cells grow locally, invading surrounding tissues and cells and spread via the lymphatic and vascular systems. Malignant tumours have the particular feature of failing to achieve cellular differentiation and give rise to secondary deposits or metastases, remote from the site of origin. Malignant tumours do not arise from benign ones although normal cells may go through abnormal changes to become cancer cells (Figure 37).
One abnormal change not shown above is metaplasia and this may be a first step towards neoplasia. Metaplasia is not a normal physiological process and refers to the replacement of one cell type with another in the same tissue (Figure 38). Cells that appear normal may also become cancerous by way of abnormal changes such as hyperplasia and dysplasia. Hyperplasia is a common pre-neoplastic response to a stimulus that results in an increase in the reproduction rate of normal looking cells. In dysplasia however, while the cells may have some of the cytological features of malignancy they are not cancerous (Figure 39). Although hyperplasia and dysplasia are often initial stages in the development of cancer, they are both reversible.
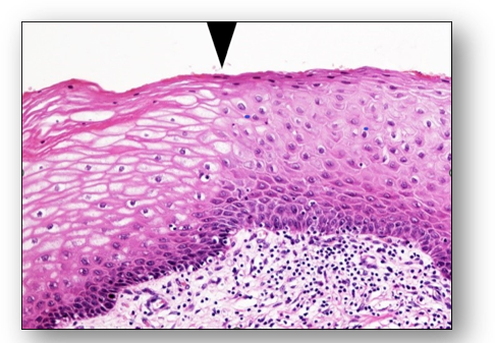
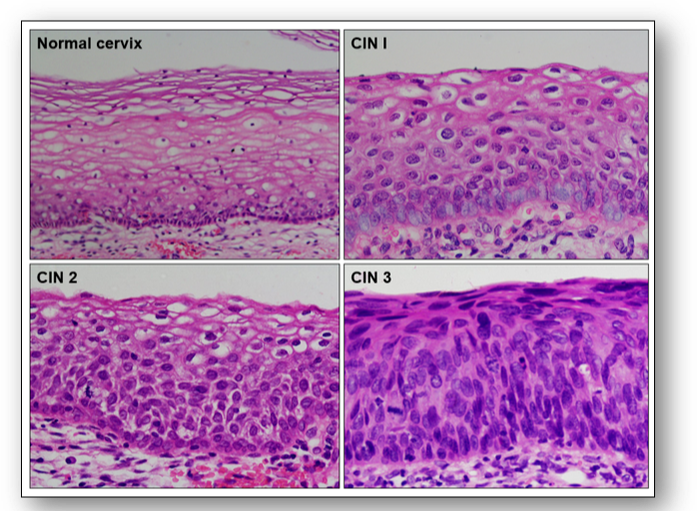
One of the most severe types of premalignant change is the condition termed carcinoma in situ where abnormal cells remain within the epithelium. Since the cells have not breached the basement membrane, they have not invaded neighbouring tissue. However, carcinoma in situ remains a vivid reminder of the need for action since it has a high risk of developing into an invasive malignancy. In the cervix, this is referred to as cervical intraepithelial neoplasia (CIN) and the grade is dependent upon the depth of epithelium involved (Figure 40).
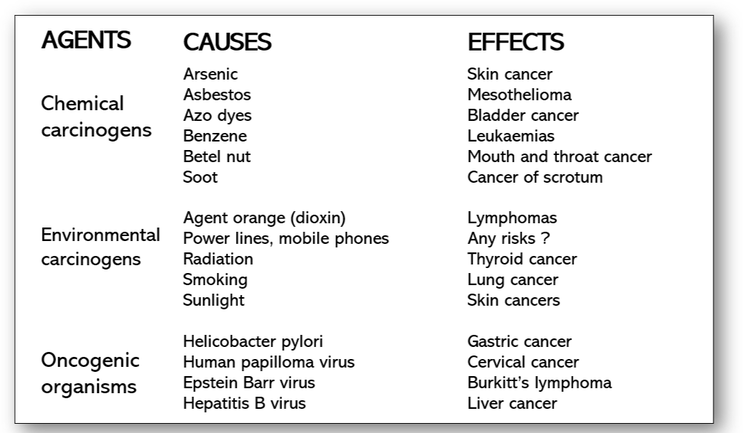
Cancer is a disease that is caused by changes to genes that control the way our cells function, grow and divide. Cancer cells are not only resistant to signals that promote cell death (apoptosis) but are able to multiply in the absence of growth factors usually required for the proliferation of normal cells. Cells become cancerous following an increase in mutations, particularly in the growth promoting genes that are responsible for controlling cellular division and proliferation. In order for these mutations to induce cancer, they must occur in dividing cells so that they may be passed on. Mutations such as those often inherited genetically from parents can also occur during lifetime as a consequence of environmental agents such as tobacco smoke and ultraviolet radiation.
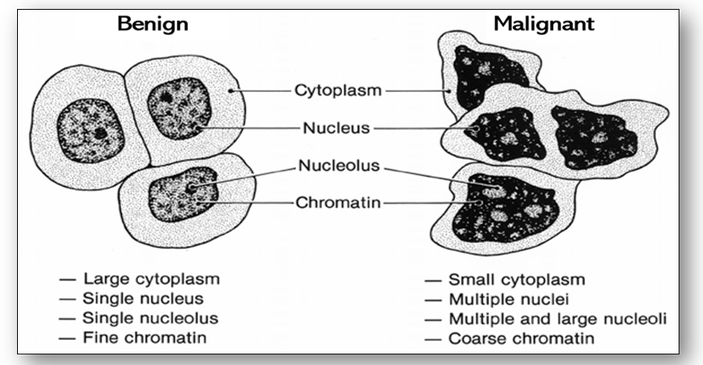
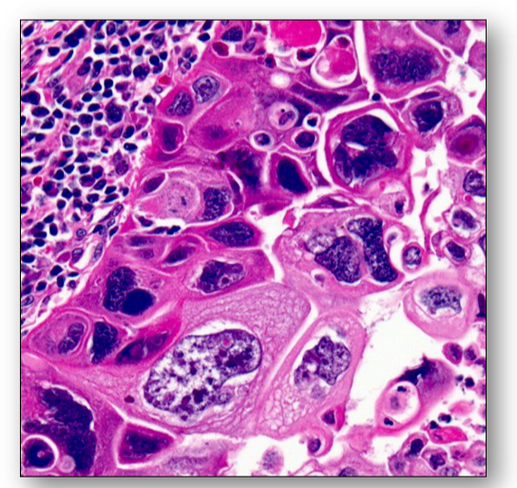
Cancer cells can be distinguished from normal and benign cells by microscopic examination (see Figure 36 above). Unlike benign tumours, malignant cells have an increased variation in nuclear and cell size and shape (pleomorphism) and in the density of nuclear staining (hyperchromasia). They also have an atypical cytology with an increase in the nuclear to cytoplasmic ratio and are usually less well differentiated than normal or benign tumour cells (Figure 41).
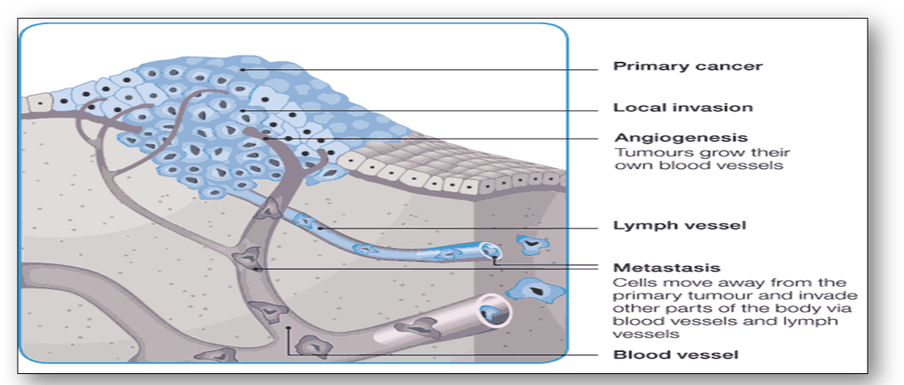
Malignant tumours often have poorly defined margins with no obvious capsule. Cancer cells also invade surrounding tissues by breaking through the basal laminas that define the boundaries of tissues. By producing proteases, they allow invasion of these tissues and often metastasize to distant sites via the vascular and lymphatic systems (Figure 42). Without a suitable blood supply tumours are unable to spread effectively. However, by stimulating the secretion of a tumour angiogenesis factor, cancers have the ability to grow their own blood vessels. Nonetheless, production of new vessels is often unable to keep up with increased cellular proliferation and cell death or necrosis is frequently seen central to the tumour.
Grading and staging of tumours
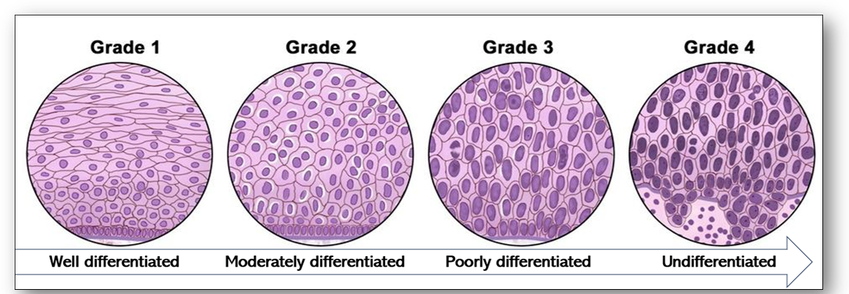
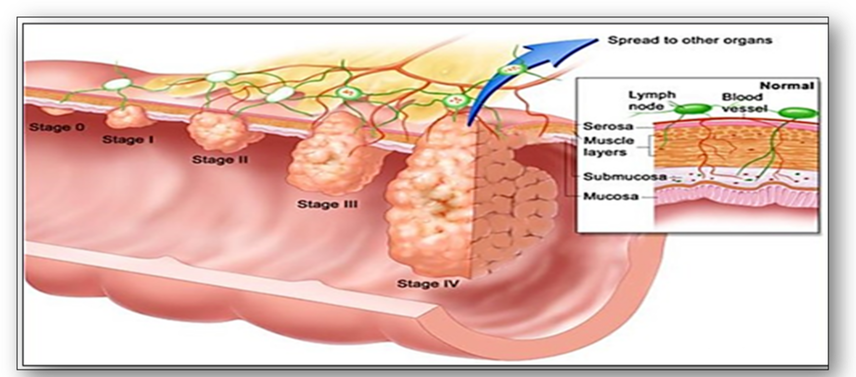
When cancerous tumours are diagnosed in the histology laboratory, their cellular appearance (grading) and extent (staging) are assessed whenever possible. Grading of tumours is a way of classifying cancer cells microscopically and is dependent upon what the cancer cells look like when compared with normal cells (Figure 43). Tumours are usually described as low or high grade based on their degree of differentiation, how quickly they are growing and dividing and how likely they are to spread. Conversely, staging of tumours is dependent upon several factors such as size of tumour and its distribution (Figure 44). In summary, the higher the stage, the larger the tumour and the farther it has spread.
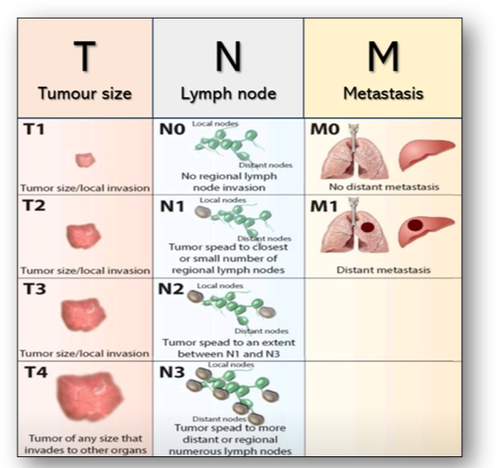
In the staging of bowel cancer for example, stage O signifies carcinoma in situ where the cancer cells remain in the epithelium and have yet to penetrate the basement membrane (Figure 44). Stage I is when the tumour is small, has invaded the surrounding tissues but has not spread. If the tumour becomes larger, it is classed as stage II, but once the tumour spreads to local lymph nodes, it becomes stage III. However, stage IV indicates cancer has spread beyond its original location to distant lymph nodes and formed metastases in other tissues and organs. The ability of malignant tumours to metastasize and invade normal tissues makes cancer such a difficult disease to treat. Another commonly adopted staging classification is the TNM staging system where T = size of tumour, N = number of lymph nodes involved and M = metastatic spread (Figure 45).
There are other grading and staging systems in current use. Traditionally, prostate cancer grades were described according to the Gleason score and breast cancer grades identified using the Scarff-Bloom-Richardson system. Similarly in staging, although the most common system for bowel cancer is the TNM system outlined above, Duke’s classification using A,B,C and D as the grades is also often used in bowel cancers.
6. Classification of tumours
Tumours are based on the tissues of origin and can be broadly subdivided as epithelial and non-epithelial (mesenchymal) tumours.
Tumours of epithelium
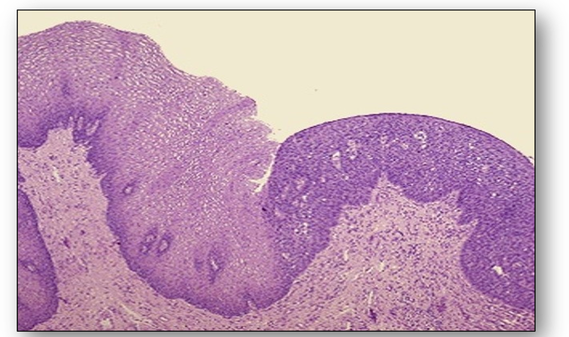
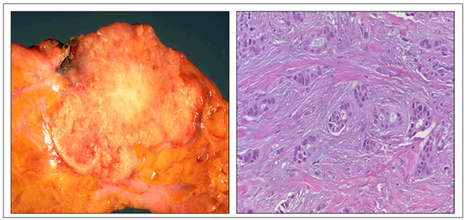
Benign tumours of squamous origin are termed papillomas and benign tumours of glandular origin are termed adenomas (Figure 47). Most common of these are papillomas of the skin and adenomatous polyps such as those found in the bowel. Benign neoplasms do not generally give rise to malignant ones. Malignant tumours of squamous origin are termed squamous carcinomas (which include basal cell carcinomas and malignant melanomas), and those of glandular origin are termed adenocarcinomas (Figure 48). A basal cell carcinoma and a malignant melanoma which is larger and more pigmented can be seen in Figure 49 and a ductal carcinoma of the breast showing the carcinoma infiltrating through the breast stroma in Figure 50.
|
|
|
Non-epithelial tumours
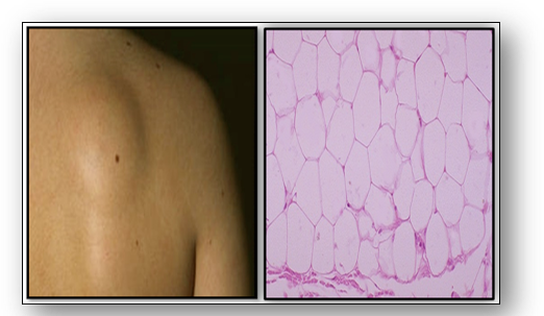
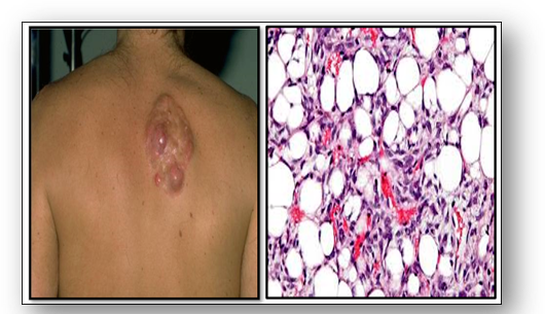
Benign mesenchymal tumours usually have the suffix ‘-oma’ and the malignant tumours ‘-sarcoma’ (see Figure 46). Microscopy of benign tumours such as a lipoma show resemblance to normal tissue (Figure 51). However, microscopy of a malignant liposarcoma shows the cancer cells distributed throughout the adipose tissue (Figure 52).
Non-epithelial tumours
Benign mesenchymal tumours usually have the suffix ‘-oma’ and the malignant tumours ‘-sarcoma’ (see Figure 46). Microscopy of benign tumours such as a lipoma show resemblance to normal tissue (Figure 51). However, microscopy of a malignant liposarcoma shows the cancer cells distributed throughout the adipose tissue (Figure 52).
|
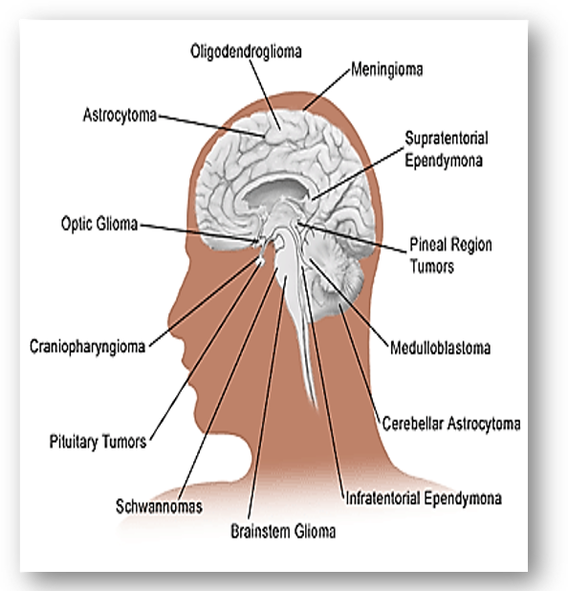
In the nervous system, connective tissue nerve cells are collectively known as glia which are further subdivided into astrocytes, ependymal cells, and oligodendroglia. Tumours arising from glia are termed gliomas and are the most prevalent type of malignant brain tumour in adults (Figure 53). In children, the most common malignant brain tumour is a medulloblastoma, a rapidly growing, nerve cell tumours of the cerebellum, while neuroblastoma, another aggressive malignant tumour develops from immature nerve cells. They are found in several areas of the body and most commonly arises in and around the adrenal glands, which have similar origins to nerve cells. Nerve tumours are abnormal masses that grow on or in peripheral nerves that branch from the brain and spinal cord through the rest of the body. The most common type of benign peripheral nerve tumour in adults is the schwannoma, a tumour of the nerve sheath. Also, ganglioneuromas are rare tumours of ganglion cells and fibres that usually behave as benign tumours. They often start in autonomic nerve cells which manage bodily functions such as blood pressure. |
3. Other tumour types
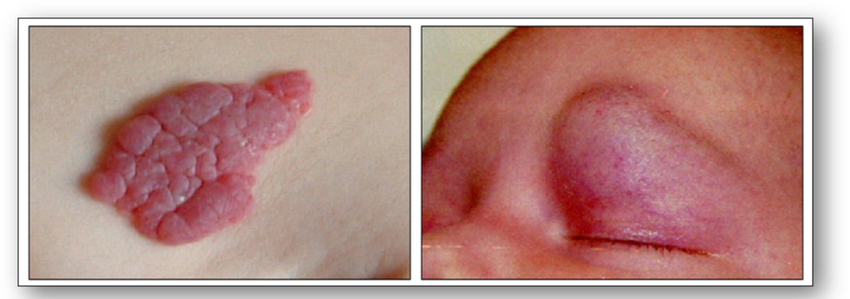
Benign angiomas (hamartomas) are not true tumours and usually comprise of masses of vascular tissue (Figure 54). A naevus (mole or birthmark) is a hamartoma of the skin and this may be pigmented.
- Blood vessels and lymphatics
Benign angiomas (hamartomas) are not true tumours and usually comprise of masses of vascular tissue (Figure 54). A naevus (mole or birthmark) is a hamartoma of the skin and this may be pigmented.
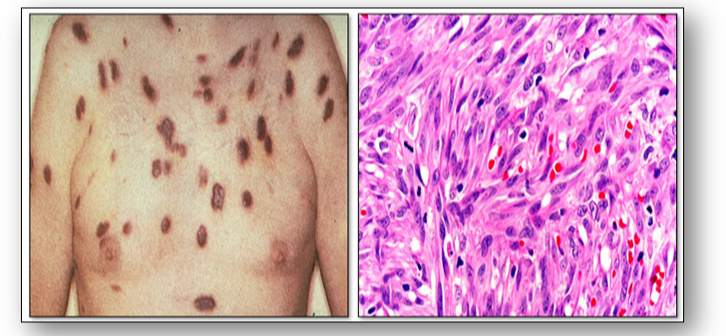
Kaposi sarcoma is a malignant vascular tumour which is characterised by multiple purple patches or nodules on the skin and / or mucous membranes that line the gastrointestinal tract (see Figure 55). The tumour is caused by a human herpes virus and typically occurs in patients between the ages of 50-70 years. The classic, endemic form is related to prolonged immunosuppression therapy and generally occurs on the skin in renal transplant patients. The epidemic type of Kaposi sarcoma is AIDS-related and usually occurs in young to middle-aged adults. It can run an aggressive course and spread to lymph nodes and cause widespread systemic involvement.
The dermal proliferation in the photomicrograph of Kaposi sarcoma is comprised of a spindle cell proliferation of endothelial cells forming sinuous vascular spaces. These may be sparse in patch phase lesions, progressing to fascicles of spindle cells in nodular lesions (Figure 55).
- Lymphoid and haemopoetic tissue
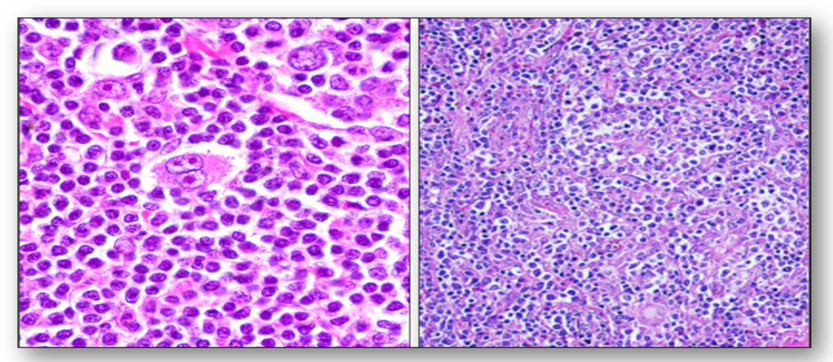
Enlarged lymph nodes (lymphadenopathy) occur as a result of infections (eg. glandular fever, tuberculosis) and tumours (eg. lymphomas). Lymphomas are primary tumours of lymph nodes. Of these, Hodgkins disease is the most common form and they contain the distinctive Reed-Sternberg cells (Figure 56). Other lymphomas are classified as non-Hodgkins lymphomas and these are subdivided into low grade and high grade. Lymphomas can also be found in other organs such as the spleen, stomach and bowel. Tumours of the blood and bone marrow are classified as leukaemias.
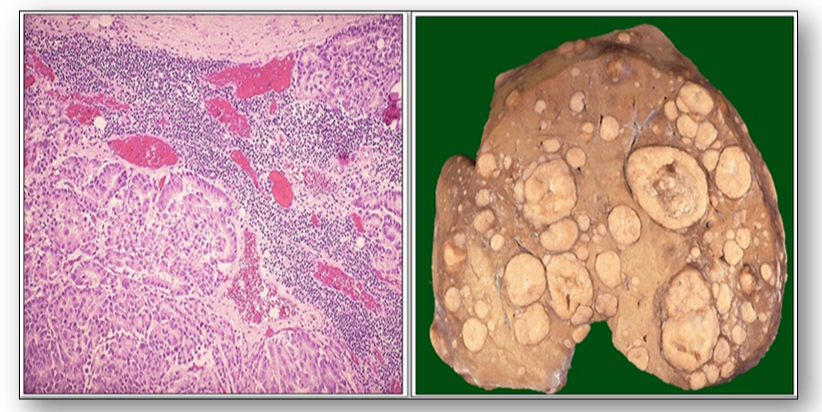
Lymph nodes also play a role in the spread of other cancers, and a secondary or metastatic tumour can occur in lymph nodes as well as in other organs (Figure 57). The image on the left shows metastatic adenocarcinoma in a lymph node. The first nodes involved are those draining the primary site and these are termed sentinel nodes. The image on the right shows multiple tan-white metastatic tumours of varying size in a liver.
Mixed tumours are formed by the collision of 2 different tumours that arise together such as adenosquamous carcinoma. Teratomas consist of various tissues, chaotically arranged with no relation to site of origin (see Figure 58a). They are not rare and are usually found in the ovaries and testes. They contain cartilage, bone, epithelia, hair and teeth in the benign form, which appear to be derived from germ cells. Mature teratomas are the most common type of ovarian germ cell tumour. They are non cancerous and often called dermoid cysts (see below). Seminoma is the commonest malignancy of the testis. Of germ cell origin, it is normally found between the ages of 20-50 years. Choriocarcinoma is a highly malignant tumour that originates from trophoblasts. |
Cysts
These are not tumours but are listed here for convenience. The term cyst properly means a space containing fluid and lined by epithelial cells. In most cysts, the lining epithelium is not neoplastic and these cysts are neither tumours nor parts of tumours (see teratoma above). Nearly all cysts arise by the abnormal dilatation of pre-existing tubules, ducts or cavities, though a cyst may lose its cell lining due to inflammatory or other change.
Congenital cysts are divided into 2 types. Firstly, those that arise within otherwise normal organs or tissues such as in the genitourinary tract (where small cysts are common) and branchial cysts, which are found at the side of the neck. Secondly, cysts arising as part of a major congenital abnormality of an organ such as polycystic disease of the kidney (Figure 58b).
Acquired cysts are further subdivided into 3 types:
These are not tumours but are listed here for convenience. The term cyst properly means a space containing fluid and lined by epithelial cells. In most cysts, the lining epithelium is not neoplastic and these cysts are neither tumours nor parts of tumours (see teratoma above). Nearly all cysts arise by the abnormal dilatation of pre-existing tubules, ducts or cavities, though a cyst may lose its cell lining due to inflammatory or other change.
Congenital cysts are divided into 2 types. Firstly, those that arise within otherwise normal organs or tissues such as in the genitourinary tract (where small cysts are common) and branchial cysts, which are found at the side of the neck. Secondly, cysts arising as part of a major congenital abnormality of an organ such as polycystic disease of the kidney (Figure 58b).
Acquired cysts are further subdivided into 3 types:
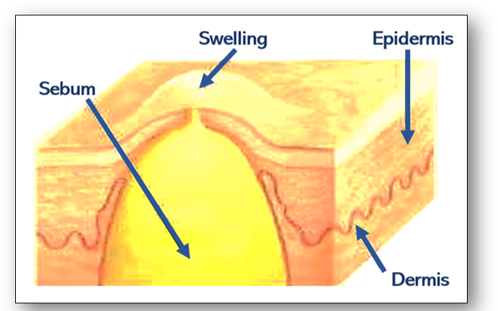
- Retention cysts are formed by the retention of secretion produced by obstruction of the lumen of a duct. For example, when a sebaceous gland containing oily sebum becomes blocked, it fills with fatty material and forms a sebaceous cyst (Figure 58c).
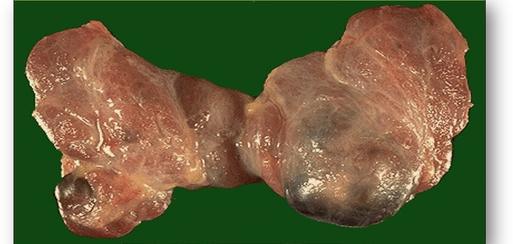
- Distension cysts are formed from natural enclosed spaces such as in the thyroid gland (Figure 58d).
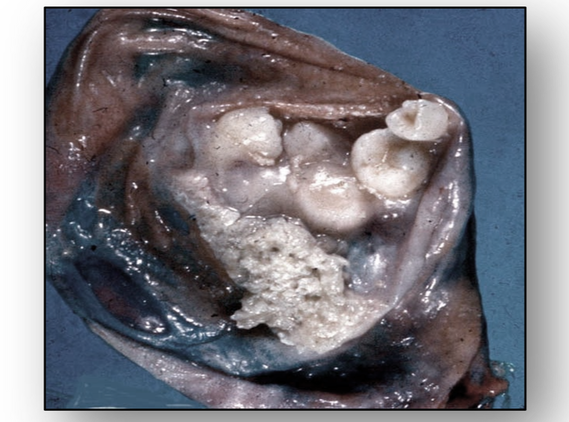
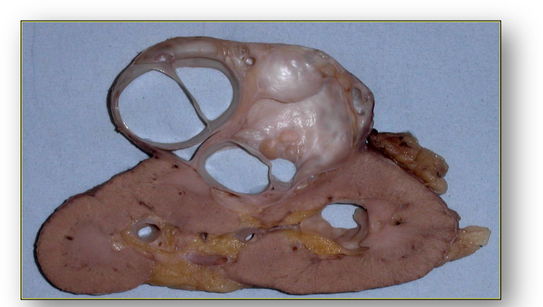
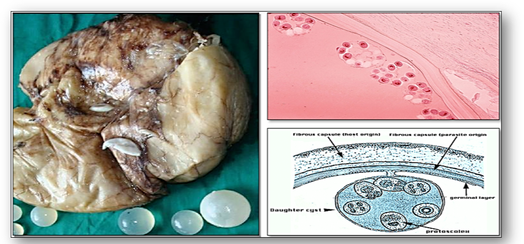
- Parasitic cysts, such as hydatid cysts, are cystic stages in the life cycle of the parasitic dog tapeworm Taenia echinococcus and are usually found in the liver (Figure 58e). Also see section on cestodes in the chapter on infections.
7. Multidisciplinary team meetings (MDTs)
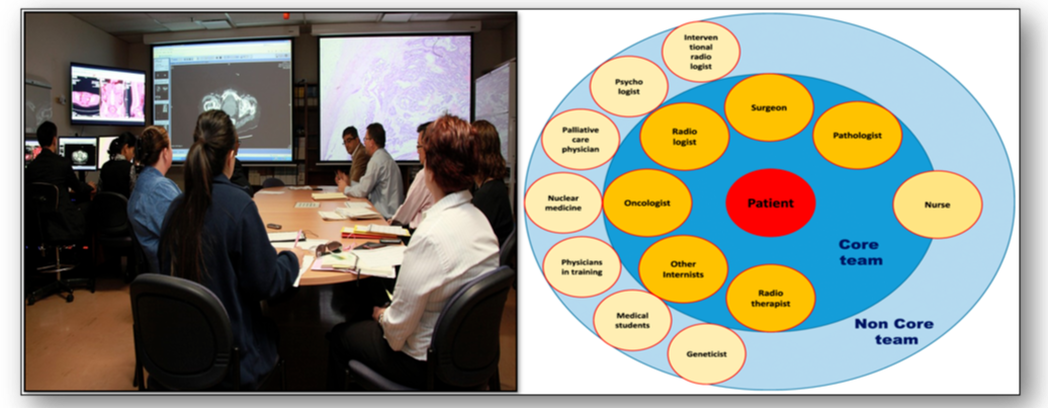
An MDT is group of professionals from one or more clinical disciplines who together discuss and recommend treatments for individual patients in certain conditions such as cancer (Figure 59). Clinical decisions are made based on reviews of clinical documentation such as pathology results and diagnostic imaging. In this way, MDTs are able to focus on patient needs and increase the efficiency and effectiveness of medical treatments. Through strong leadership, meetings can be arranged, action plans developed and senior personnel informed of all developments. Clear communication channels provide a coordinated opportunity to learn from one another, avoiding duplication of workload and saving resources. However, personal commitment, logistics of arranging meetings, voice equality and support from organisations for protected time remain potential pitfalls.
Diagnostic methods for cancer
Physical examination
Medical history, signs, symptoms and direct patient examination can often identify underlying tumours which may be visible or palpable.
Radiography
The use of X rays, computed tomography (CT), magnetic resonance imaging (MRI), mammography, positron emission tomography (PET) scans and ultrasound may all be helpful in detecting the presence and location of tumours. Subsequent sampling can aid diagnosis, grading and staging of a cancer and often determine therapy.
Laboratory investigations
General findings from haemoglobin and full blood counts such as anaemia and haematuria are helpful to suggest further analysis. High or low levels of certain substances can be a sign of cancer and biochemical testing of blood, urine, and other body fluids may also aid diagnosis. More specific testing such as prostate specific antigen (PSA) levels may help to determine the presence of specific tumours. Cytogenetic analysis may be used to help diagnose cancer and plan treatment, and genetic testing for specific genes (such as BRCA-1 for breast cancer) may suggest an increased risk for some malignancies.
Histology
Methods that sample small pieces of tissue (biopsies) from a particular site, often via endoscopic and needle techniques can often yield specific information regarding a diagnosis of malignancy. Surgical removal of tissue samples can also help to determine the stage and grade of tumour. Immunohistochemical testing for certain markers produced by cancer cells and liquid biopsy samples can also be useful in identifying proteins and genetic material from cancers. These methods often help in early diagnosis and are useful in planning treatment, measuring how well the treatment is working, and watch for signs of tumour recurrence.
Cytology
Methods for sampling cells can be simple, cost effective and minimally invasive. A good example is the cervical smear for diagnosis of cervical abnormalities and cancers. Cells exfoliated into body fluids may also be examined. Fine needle aspiration (FNA) can be used to sample a variety of lesions. Sputum and urine analysis also play a role in detecting abnormal cells from the lungs and urinary tract.
Post mortems
Tumours are not always detected or diagnosed during life and the autopsy can serve as a clinical diagnostic method and help gather information regarding diagnosis and treatment of cancers for future research.
8. Autoimmune disorders
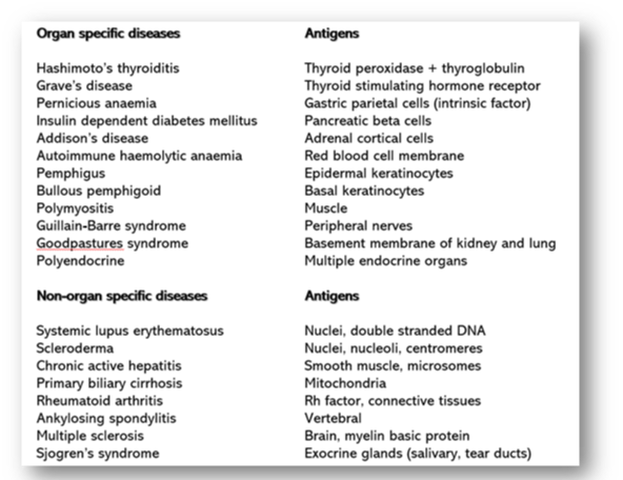
Autoimmune diseases are the result of the body producing an immune response against its tissue components. In these diseases, it is recognised that the normal mechanisms ensuring tolerance for self-antigens have broken down. In some situations, there may be a genetic component, whilst in others, autoimmune diseases can be triggered by a viral or microbial infection. In these instances, the organisms carry cross-reacting antigens that show molecular mimicry to human tissues. Responses may be directed against a single component of a single tissue (organ specific), as seen in Hashimoto’s disease of the thyroid. However, in most cases, the response is against a tissue component present in many tissues and organs throughout the body (non-organ specific), as found in connective tissue disorders such as systemic lupus erythematosus (Figure 60).
Autoimmune diseases are the result of the body producing an immune response against its tissue components. In these diseases, it is recognised that the normal mechanisms ensuring tolerance for self-antigens have broken down. In some situations, there may be a genetic component, whilst in others, autoimmune diseases can be triggered by a viral or microbial infection. In these instances, the organisms carry cross-reacting antigens that show molecular mimicry to human tissues. Responses may be directed against a single component of a single tissue (organ specific), as seen in Hashimoto’s disease of the thyroid. However, in most cases, the response is against a tissue component present in many tissues and organs throughout the body (non-organ specific), as found in connective tissue disorders such as systemic lupus erythematosus (Figure 60).
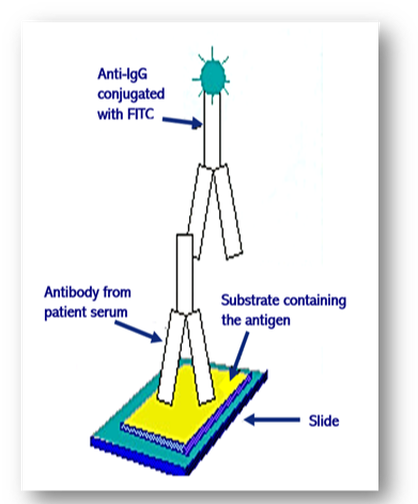
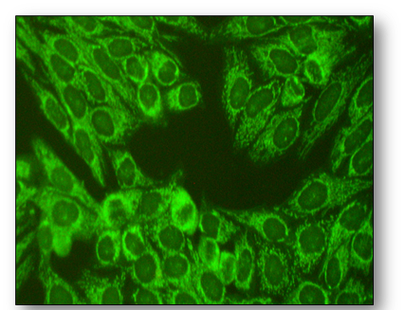
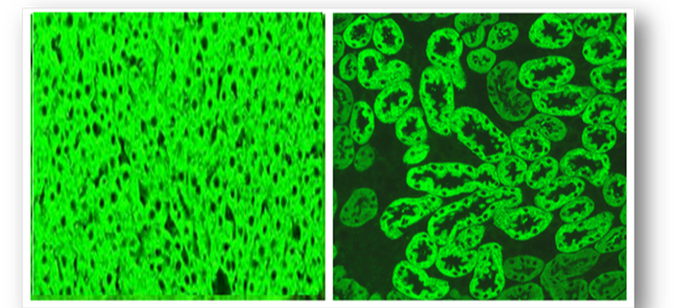
Introduced in 1957, these methods have largely remain unchanged and are an essential part of clinical medicine. Since autoantibodies are often found in the blood, they can be detected simply and reliably using rodent tissues or Hep-2 cells as substrate (see below). In this indirect immunofluorescent method, diluted patient serum is incubated on the substrate tissues and washed in buffer (Figure 61). Further incubation with a fluorescein isothiocyanate (FITC) conjugated anti-human IgG antibody produces apple green fluorescence in positive samples when viewed with a fluorescent microscope. Alternatively, tissue from the patient can be used as substrate (direct method).
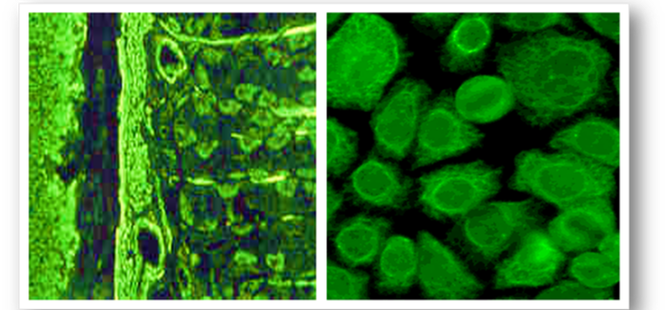
Presently, tissue sections and Hep-2 cells allow pattern recognition of over a 100 different autoantibodies. Rodent liver, kidney and stomach (LKS) are traditional substrates, are widely available and the least expensive of the animal tissues. Human epithelioma type 2 (Hep-2) cells are immortalised cells that originate from human laryngeal carcinoma and are grown as monolayers on slides. Hep-2 cells are a sensitive substrate that allows identification of many autoantibodies and because they are of human origin, better specificity is ensured. The cell monolayers also ensure that no obscuring cellular matrix is present and that all nuclei are visible, allowing more complex staining patterns to be seen (Figure 62). The antigen distribution is uniform and because cell division rates are higher, antigens produced during this phase are easily located. By using rodent LKS tissues or Hep-2 cells routinely, the detection of several important antibodies can be identified (Figure 63). When interpreting staining patterns, the intensity of fluorescence should be compared to that of known controls and calculation of end points can be achieved if required by using serial dilutions of patient serum. However, it must be remembered that fluorescence is partly subjective and variation in interpretation of sample may occur between individual screeners. Low titres of some antibodies can also be found in normal people and in relatives of patients with autoimmune disease or cancer. Titres are not indicators of disease progression and low or absent titres do not necessarily exclude disease. Subscription to an external quality assessment scheme (EQA) provide comparisons of methods of laboratory testing and are generally mandatory for accreditation purposes.
Antinuclear antibodies (ANA)
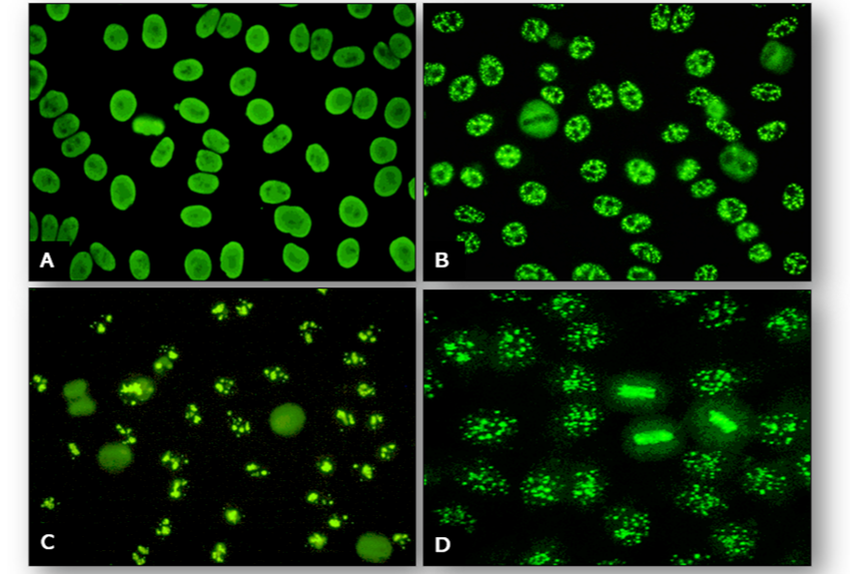
Although rodent LKS tissues can be used for demonstration of ANA, tests are best performed using Hep-2 cells as several patterns can be identified. The four common patterns are homogenous, speckled, nucleolar and centromere (Figure 64).
Associated antigens : dsDNA, histones
Associated diseases : SLE, Rheumatoid arthritis, Systemic sclerosis (scleroderma)
Associated antigens : RNP, Sm, SS-A (Ro), SS-B (La)
Associated diseases : SLE (99% specific, 20% patients), Polymyositis, Rheumatoid arthritis, MCTD, Sjogren’s syndrome
Associated antigens : fibrillarin, Scl, RNA polymerase
Associated diseases : Systemic sclerosis (5%), Scleroderma / Polymyositis (50%), Raynaud’s disease
Associated antigens : kinetochore proteins
Associated diseases : CREST syndrome in 60% of patients (Calcinosis, Raynaud’s, Esophageal dysmotility, Sclerodactyly and Telangiectasis)
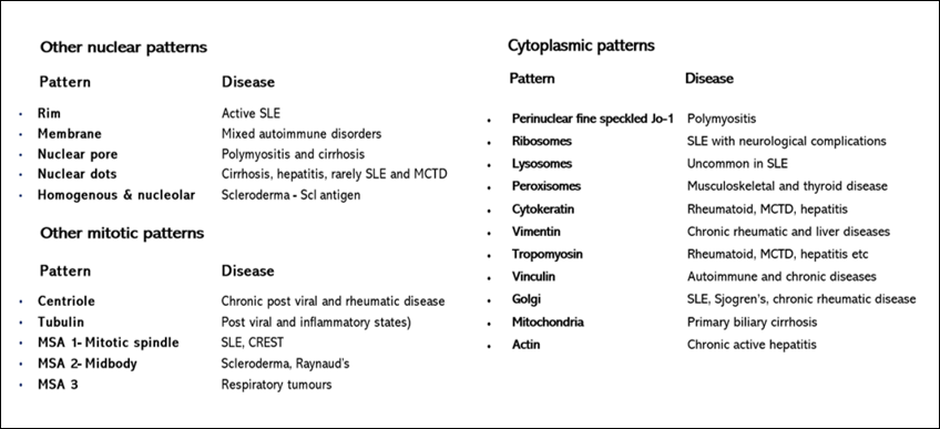
In addition, there are many other patterns that may be seen occasionally and these are listed below (Figure 65).
- Homogenous pattern
Associated antigens : dsDNA, histones
Associated diseases : SLE, Rheumatoid arthritis, Systemic sclerosis (scleroderma)
- Speckled pattern
Associated antigens : RNP, Sm, SS-A (Ro), SS-B (La)
Associated diseases : SLE (99% specific, 20% patients), Polymyositis, Rheumatoid arthritis, MCTD, Sjogren’s syndrome
- Nucleolar pattern
Associated antigens : fibrillarin, Scl, RNA polymerase
Associated diseases : Systemic sclerosis (5%), Scleroderma / Polymyositis (50%), Raynaud’s disease
- Centromere pattern
Associated antigens : kinetochore proteins
Associated diseases : CREST syndrome in 60% of patients (Calcinosis, Raynaud’s, Esophageal dysmotility, Sclerodactyly and Telangiectasis)
In addition, there are many other patterns that may be seen occasionally and these are listed below (Figure 65).
DNA antibodies
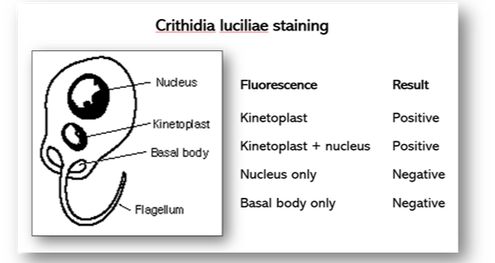
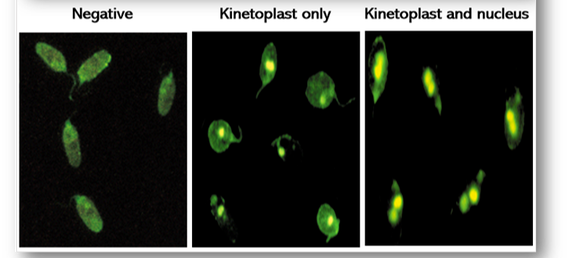
In the event of a positive antinuclear test, antibodies to double stranded DNA are performed on the patient’s serum to differentiate between SLE and other collagen diseases. The antigen used is the haemoflagellate Crithidia luciliae which contains a giant mitochondrion or kinetoplast (Figures 66). A positive result occurs when the kinetoplast stains alone or with the nucleus. If fluorescence is seen in the basal body or nucleus alone, the results are negative for DNA.
Mitochondrial antibodies
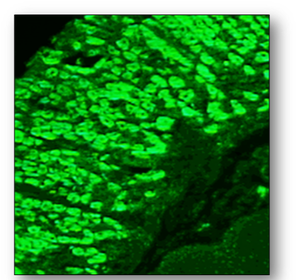
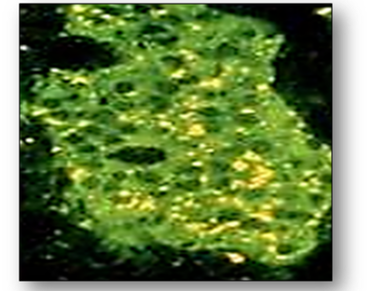
This antibody is the hallmark of primary biliary cirrhosis which may also be associated with several other disorders such as Raynaud’s disease, Sjogren’s syndrome and scleroderma. Positive samples show a granular cytoplasmic fluorescence in LKS tissues or Hep-2 cells (Figure 67).
Smooth muscle antibodies (SMA)
These are associated with autoimmune hepatitis and can be demonstrated with rodent liver and kidney tissues. These antibodies are also visible as intracytoplasmic fibres on Hep-2 cells (Figure 68).
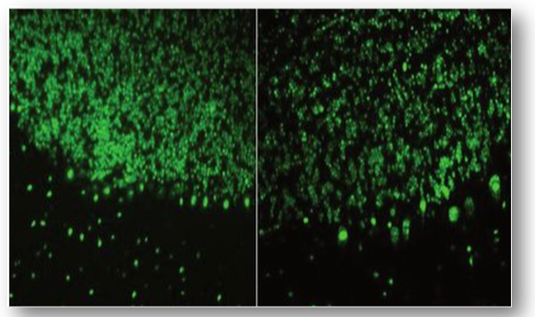
Parietal cell antibodies (PCA)
Gastric parietal cell antibodies are typically found in a high percentage of patients with pernicious anaemia (Figure 69). The antibodies are not disease specific and can be detected in patients with chronic gastritis, Sjogren's syndrome and other autoimmune disorders.
Antibodies to kidney
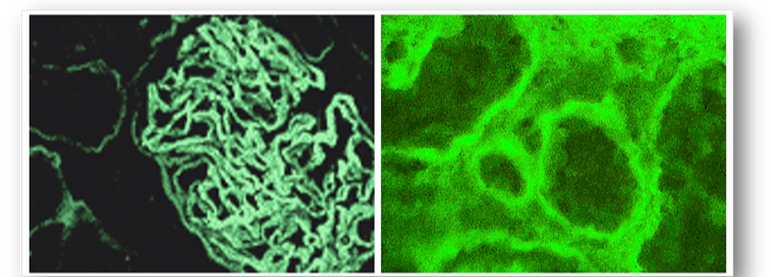
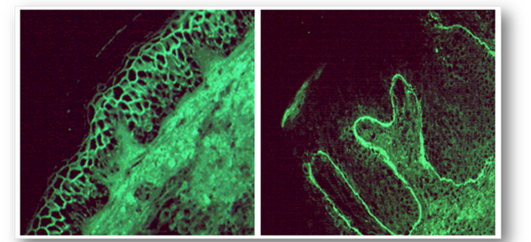
- Glomerular basement membrane (GBM) antibodies (Figure 70)
- Tubular basement membrane antibodies (Figure 70)
Liver / kidney microsomal (LKM) antibodies
Although these antibodies are not commonly found in chronic active hepatitis, the test is primarily carried out with tests for smooth muscle and antinuclear antibodies. These combined tests help to diagnose and differentiate type 1 autoimmune hepatitis from type 2. Positive results show homogenous staining of liver hepatocytes & renal tubules (Figure 71).
Other liver antibodies which may be found autoimmune conditions include liver membrane antibody, liver specific membrane lipoprotein, liver cytosolic antigen type I antibody, soluble liver antigen, bile duct and bile canalicular antibodies.
Antibodies to endocrine tissue
- Thyroid antibodies
Antibodies to intestinal tissue
- Antibodies to pancreas
Antibodies to intestinal tissue
- Endomysial antibodies
Antibodies to skin
Patient’s serum (or skin) can be used in the diagnosis of primary bullous dermatoses (see Figure 75).
- Pemphigus
- Pemphigoid
- Dermatitis herpetiformis
Antibodies to neurological tissue
- Purkinje cell antibodies
- Anti neurone nuclear antibodies (ANNA)
Extractable nuclear antigens (ENA)
ENAs are targets for autoantibodies that react with proteins in cell nuclei. Although there are multiple different antigens, only 6 are tested routinely and they are described below.
- Sm antigen - This gives a coarse speckled nuclear pattern and is diagnostic of SLE.
- U1-snRNP - This small nuclear ribonucleoprotein particle (snRNP) is a target of autoreactive B cells and T cells in several rheumatic diseases such as SLE and mixed connective tissue disease (MCTD).
- SS-A (Ro) - This produces a fine speckled pattern and is associated with SS-B in Sjogren’s syndrome.
- SS-B (La) - This produces a fine speckled, grainy pattern and is associated with SS-A.
- Scl-70 - This is seen as an homogenous or fine speckled pattern with nucleolar and is frequently found in systemic sclerosis (scleroderma).
- Jo-1 - This produces a fine granular perinuclear pattern and is highly specific for polymyositis.
Anti neutrophil cytoplasmic antibodies (ANCA)
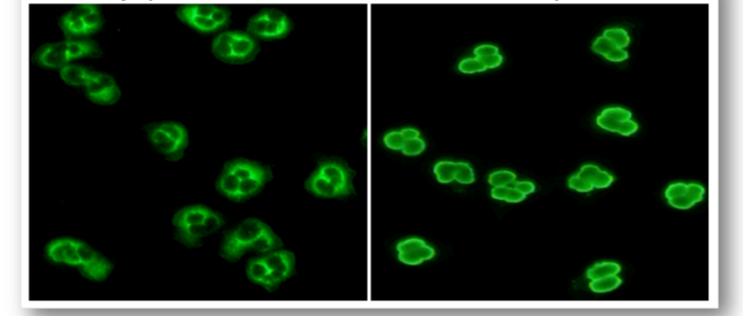
These are a heterogeneous group of rare autoimmune conditions that cause inflammation of blood vessels with various manifestations. They are used to assess vascular disorders such as Wegener’s granulomatosis and systemic vasculitis. The laboratory test uses a neutrophil substrate which help to distinguish c-ANCA from p-ANCA (Figure 79).
In c-ANCA, the target antigen is proteinase 3 (PR3) and exhibits cytoplasmic staining in patients with Wegener’s granulomatosis.
In p-ANCA, the target antigen is myeloperoxidase (MPO) and produces perinuclear staining in patients with systemic vasculitis.
Website last reviewed on 4th July 2024